Amivantamab efficacy in wild-type EGFR NSCLC tumors correlates with levels of ligand expression
- PMID: 39242834
- PMCID: PMC11379809
- DOI: 10.1038/s41698-024-00682-y
Amivantamab efficacy in wild-type EGFR NSCLC tumors correlates with levels of ligand expression
Abstract
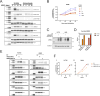
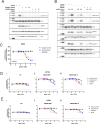
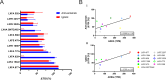
Amivantamab is an FDA-approved bispecific antibody targeting EGF and Met receptors, with clinical activity against EGFR mutant non-small cell lung cancer (NSCLC). Amivantamab efficacy has been demonstrated to be linked to three mechanisms of action (MOA): immune cell-mediated killing, receptor internalization and degradation, and inhibition of ligand binding to both EGFR and Met receptors. Among the EGFR ligands, we demonstrated that amphiregulin (AREG) is highly expressed in wild-type (WT) EGFR (EGFRWT) NSCLC primary tumors, with significantly higher circulating protein levels in NSCLC patients than in healthy volunteers. Treatment of AREG-stimulated EGFRWT cells/tumors with amivantamab or with an AREG-targeting antibody inhibited ligand-induced signaling and cell/tumor proliferation/growth. Across 11 EGFRWT NSCLC patient-derived xenograft models, amivantamab efficacy correlated with AREG RNA levels. Interestingly, in these models, amivantamab anti-tumor activity was independent of Fc engagement with immune cells, suggesting that, in this context, the ligand-blocking function is sufficient for amivantamab maximal efficacy. Finally, we demonstrated that in lung adenocarcinoma patients, high expression of AREG and EGFR mutations were mutually exclusive. In conclusion, these data 1) highlight EGFR ligand AREG as a driver of tumor growth in some EGFRWT NSCLC models, 2) illustrate the preclinical efficacy of amivantamab in ligand-driven EGFRWT NSCLC, and 3) identify AREG as a potential predictive biomarker for amivantamab activity in EGFRWT NSCLC.
© 2024. The Author(s).
Conflict of interest statement
R.R.S., B.H., S.L., H.G., M.W., S.V., T.S.Y. are current employees of Johnson & Johnson but declare no non-financial competing interests. S.L. and S.M. are former employees of Johnson & Johnson but declare no non-financial competing interests. MP and KPV declare no competing interests. S.M. and B.H. reports a patent application for US17/687,984 - Treatment of Cancers Lacking EGFR- Activating Mutations, pending. S.M reports a patent US9593164B2 - bispecific EGFR/Met antibodies, issued.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

