Roles of microbiota in autoimmunity in Arabidopsis leaves
- PMID: 39242981
- PMCID: PMC11410663
- DOI: 10.1038/s41477-024-01779-9
Roles of microbiota in autoimmunity in Arabidopsis leaves
Abstract
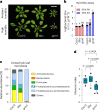
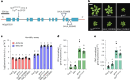
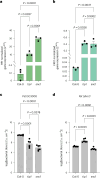
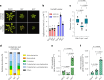
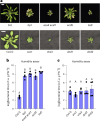
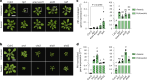
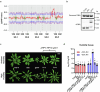
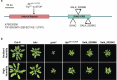
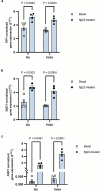
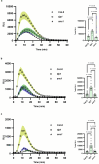
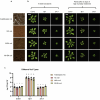
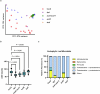
Over the past three decades, researchers have isolated plant mutants that show constitutively activated defence responses in the absence of pathogen infection. These mutants are called autoimmune mutants and are typically dwarf and/or bearing chlorotic/necrotic lesions. Here, from a genetic screen for Arabidopsis genes involved in maintaining a normal leaf microbiota, we identified TIP GROWTH DEFECTIVE 1 (TIP1), which encodes an S-acyltransferase, as a key player in guarding leaves against abnormal microbiota level and composition under high-humidity conditions. The tip1 mutant has several characteristic phenotypes of classical autoimmune mutants, including a dwarf stature, showing lesions, and having a high basal level of defence gene expression. Gnotobiotic experiments revealed that the autoimmune phenotypes of the tip1 mutant are largely dependent on the presence of microbiota as axenic tip1 plants have markedly reduced autoimmune phenotypes. We found that the microbiota dependency of autoimmune phenotypes is shared by several 'lesion mimic'-type autoimmune mutants in Arabidopsis. It is worth noting that autoimmune phenotypes caused by mutations in two Nucleotide-Binding, Leucine-Rich Repeat (NLR) genes do not require the presence of microbiota and can even be partially alleviated by microbiota. Our results therefore suggest the existence of at least two classes of autoimmunity (microbiota-dependent versus microbiota-independent) in plants. The observed interplay between autoimmunity and microbiota in the lesion mimic class of autoimmunity is reminiscent of the interactions between autoimmunity and dysbiosis in the animal kingdom. These parallels highlight the intricate relationship between host immunity and microbial communities across various biological systems.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Update of
-
Roles of microbiota in autoimmunity in Arabidopsis.bioRxiv [Preprint]. 2023 Jul 4:2023.03.06.531303. doi: 10.1101/2023.03.06.531303. bioRxiv. 2023. Update in: Nat Plants. 2024 Sep;10(9):1363-1376. doi: 10.1038/s41477-024-01779-9. PMID: 36945461 Free PMC article. Updated. Preprint.
Comment in
-
Unbalanced leaf microbiota can cause autoimmunity in plants.Nat Plants. 2024 Sep;10(9):1291-1292. doi: 10.1038/s41477-024-01782-0. Nat Plants. 2024. PMID: 39242984 No abstract available.
References
-
- Staskawicz, B. J., Ausubel, F. M., Baker, B. J., Ellis, J. G. & Jones, J. D. Molecular genetics of plant disease resistance. Science268, 661–667 (1995). - PubMed
-
- Jones, J. D. & Dangl, J. L. The plant immune system. Nature444, 323–329 (2006). - PubMed
-
- Lewis, J. D., Guttman, D. S. & Desveaux, D. The targeting of plant cellular systems by injected type III effector proteins. Semin. Cell Dev. Biol.20, 1055–1063 (2009). - PubMed
-
- Dou, D. & Zhou, J. M. Phytopathogen effectors subverting host immunity: different foes, similar battleground. Cell Host Microbe12, 484–495 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

