NAC1 promotes stemness and regulates myeloid-derived cell status in triple-negative breast cancer
- PMID: 39243032
- PMCID: PMC11378519
- DOI: 10.1186/s12943-024-02102-y
NAC1 promotes stemness and regulates myeloid-derived cell status in triple-negative breast cancer
Erratum in
-
Correction: Nac1 promotes stemness and regulates myeloid‑derived cell status in triple‑negative breast cancer.Mol Cancer. 2024 Oct 3;23(1):220. doi: 10.1186/s12943-024-02143-3. Mol Cancer. 2024. PMID: 39358788 Free PMC article. No abstract available.
Abstract
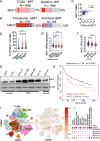
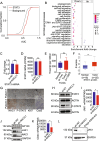
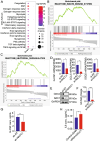
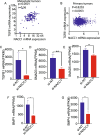
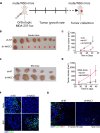
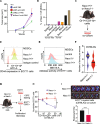
Triple negative breast cancer (TNBC) is a particularly lethal breast cancer (BC) subtype driven by cancer stem cells (CSCs) and an immunosuppressive microenvironment. Our study reveals that nucleus accumbens associated protein 1 (NAC1), a member of the BTB/POZ gene family, plays a crucial role in TNBC by maintaining tumor stemness and influencing myeloid-derived suppressor cells (MDSCs). High NAC1 expression correlates with worse TNBC prognosis. NAC1 knockdown reduced CSC markers and tumor cell proliferation, migration, and invasion. Additionally, NAC1 affects oncogenic pathways such as the CD44-JAK1-STAT3 axis and immunosuppressive signals (TGFβ, IL-6). Intriguingly, the impact of NAC1 on tumor growth varies with the host immune status, showing diminished tumorigenicity in natural killer (NK) cell-competent mice but increased tumorigenicity in NK cell-deficient ones. This highlights the important role of the host immune system in TNBC progression. In addition, high NAC1 level in MDSCs also supports TNBC stemness. Together, this study implies NAC1 as a promising therapeutic target able to simultaneously eradicate CSCs and mitigate immune evasion.
Keywords: Cancer stem cells; MDSCs; NAC1; NK cells; TME; TNBC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Wang J, et al. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444(7117):364–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

