HSF1 is a prognostic determinant and therapeutic target in intrahepatic cholangiocarcinoma
- PMID: 39243039
- PMCID: PMC11378393
- DOI: 10.1186/s13046-024-03177-7
HSF1 is a prognostic determinant and therapeutic target in intrahepatic cholangiocarcinoma
Abstract
Background: Intrahepatic cholangiocarcinoma (iCCA) is a lethal primary liver tumor characterized by clinical aggressiveness, poor prognosis, and scarce therapeutic possibilities. Therefore, new treatments are urgently needed to render this disease curable. Since cumulating evidence supports the oncogenic properties of the Heat Shock Factor 1 (HSF1) transcription factor in various cancer types, we investigated its pathogenetic and therapeutic relevance in iCCA.
Methods: Levels of HSF1 were evaluated in a vast collection of iCCA specimens. The effects of HSF1 inactivation on iCCA development in vivo were investigated using three established oncogene-driven iCCA mouse models. In addition, the impact of HSF1 suppression on tumor cells and tumor stroma was assessed in iCCA cell lines, human iCCA cancer-associated fibroblasts (hCAFs), and patient-derived organoids.
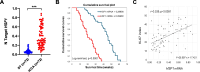
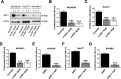
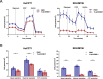
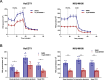
Results: Human preinvasive, invasive, and metastatic iCCAs displayed widespread HSF1 upregulation, which was associated with a dismal prognosis of the patients. In addition, hydrodynamic injection of a dominant-negative form of HSF1 (HSF1dn), which suppresses HSF1 activity, significantly delayed cholangiocarcinogenesis in AKT/NICD, AKT/YAP, and AKT/TAZ mice. In iCCA cell lines, iCCA hCAFs, and patient-derived organoids, administration of the HSF1 inhibitor KRIBB-11 significantly reduced proliferation and induced apoptosis. Cell death was profoundly augmented by concomitant administration of the Bcl-xL/Bcl2/Bcl-w inhibitor ABT-263. Furthermore, KRIBB-11 reduced mitochondrial bioenergetics and glycolysis of iCCA cells.
Conclusions: The present data underscore the critical pathogenetic, prognostic, and therapeutic role of HSF1 in cholangiocarcinogenesis.
Keywords: HSF1; Intrahepatic cholangiocarcinoma; KRIBB-11; Mouse models; Navitoclax.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261–80. 10.1038/nrgastro.2016.51 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

