Adherence and treatment discontinuation of oral semaglutide and once-weekly semaglutide injection at 12 month follow-up: Japanese real-world data
- PMID: 39243175
- PMCID: PMC11527826
- DOI: 10.1111/jdi.14265
Adherence and treatment discontinuation of oral semaglutide and once-weekly semaglutide injection at 12 month follow-up: Japanese real-world data
Abstract
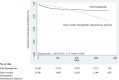
Adherence and treatment continuation rates of the glucagon-like peptide-1 receptor agonist (GLP-1RA) semaglutide for both oral (O-SEMA) and subcutaneous injection (SEMA-SC) remain unknown in real-world clinical practice. This retrospective observational study compared the 12 month adherence and treatment discontinuation of O-SEMA and once-weekly SEMA-SC in patients with type 2 diabetes using a real-world claims database. SEMA-SC initiators were 1:1 propensity score-matched to O-SEMA initiators. Non-adherence was defined as <0.8 of the proportion of days covered. SEMA-SC had a significantly higher odds ratio (OR) for non-adherence than O-SEMA (OR: 1.39). The hazard ratio for treatment discontinuation, using O-SEMA as the reference, was 1.45 for SEMA-SC, although the discontinuation rate of O-SEMA was higher during the early stage. O-SEMA initiators showed significantly higher adherence and greater persistence in therapy than SEMA-SC initiators at 12 months, which could lead to earlier initiation of GLP-1RA treatment.
Keywords: Injection; Oral; Semaglutide.
© 2024 The Author(s). Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
A.S. received lecture fees from Astellas Pharma, Inc., Eli Lilly Japan K.K., Novo Nordisk Pharma, Inc., and Sanofi K.K. The authors declare no conflicts of interest.
Approval of the research protocol: The Ethics Board of Musashino University waived the requirement for ethical approval for this study because all available data were completely anonymous with no personal information, which is characteristic of DPC‐based clinical databases. This study was conducted in accordance with the Declaration of Helsinki (revised in Fortaleza, Brazil, October 2013) and the Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Informed consent: All patient data were anonymized and did not contain personal data; therefore, informed consent was not required.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Figures

References
-
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. - PubMed
-
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double‐blind, randomised placebo‐controlled trial. Lancet 2019; 394: 121–130. - PubMed
-
- American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes – 2020. Diabetes Care 2020; 43: S98–S110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

