5-Year Outcomes of Anterior Mitral Leaflet Laceration to Prevent Outflow Obstruction
- PMID: 39243268
- PMCID: PMC11424260
- DOI: 10.1016/j.jcin.2024.05.041
5-Year Outcomes of Anterior Mitral Leaflet Laceration to Prevent Outflow Obstruction
Abstract
Background: Left ventricular outflow tract (LVOT) obstruction is a common, often fatal complication of transcatheter mitral valve replacement (TMVR). Laceration of the anterior mitral leaflet to prevent outflow obstruction (LAMPOON) was safe and effective at preventing LVOT obstruction at 30 days in the National Heart, Lung, and Blood Institute LAMPOON trial.
Objectives: The authors report the 5-year outcomes of intentional anterior mitral leaflet laceration before SAPIEN 3 TMVR, in patients at risk of LVOT obstruction.
Methods: The National Heart, Lung, and Blood Institute LAMPOON trial was a prospective, multicenter, single-arm safety and feasibility study of LAMPOON and transseptal SAPIEN 3 TMVR in annuloplasty rings (valve-in-ring) or native mitral annular calcification (MAC) (valve-in-MAC). All subjects had high predicted risk for LVOT obstruction. Subjects were not excluded for excessive frailty or comorbidity. The primary endpoints were technical success and safety at 30 days. Secondary clinical and echocardiographic endpoints were assessed at 1 year and clinical follow-up at 5 years.
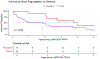
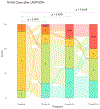
Results: Thirty subjects were enrolled between June 2017 and June 2018, equally between the valve-in-MAC and valve-in-ring arms. At 30 days, LAMPOON was successful in all 30 subjects, with no strokes, 1 (3%) death, and 1 (3%) moderate LVOT obstruction. Eighteen (65%) survived to 1 year, and 7 (25%) survived to 5 years. Six (20%) were hospitalized for heart failure in the first year. From baseline to 1 year, there was a 24-point improvement in Kansas City Cardiomyopathy Questionnaire score and a 60-m improvement in 6-minute walk distance. There was no significant change in N-terminal pro-brain natriuretic peptide. At 1 year, LVOT gradients remained low.
Conclusions: LAMPOON enabled TMVR despite the risk for LVOT obstruction. There were no long-term complications associated with LAMPOON. The selection of inoperable patients limited assessment of long-term survival following TMVR. (NHLBI DIR LAMPOON Study: Intentional Laceration of the Anterior Mitral Leaflet to Prevent Left Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Implantation; NCT03015194).
Keywords: LAMPOON; left ventricular outflow tract obstruction; mitral annular calcification; mitral valve disease; transcatheter electrosurgery; transcatheter mitral valve replacement.
Published by Elsevier Inc.
Conflict of interest statement
Funding Support and Author Disclosures This work was supported by the Division of Intramural Research, NHLBI, National Institutes of Health (Z01-HL006040), and by the intramural programs of the participating centers. The NHLBI and Edwards Lifesciences have a collaborative research and development agreement on transcatheter modification of mitral valve leaflets, including joint financial support of this study. Drs Khan, Rogers, and Lederman are coinventors on patents, assigned to the National Institutes of Health, for catheter devices to lacerate valve leaflets. Dr Khan is a proctor for Edwards Lifesciences and Medtronic; and has equity in Transmural Systems. Dr Greenbaum is a proctor for Edwards Lifesciences, Medtronic, and Abbott Vascular; and has equity in Transmural Systems and Excision Medical. Dr Babaliaros is a consultant for Edwards Lifesciences, Abbott Vascular, and Transmural Systems; and his employer has research contracts for clinical investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, St. Jude Medical, and Boston Scientific. Dr Rogers is a consultant for Edwards Lifesciences, Boston Scientific, Abbott, Anteris, and Medtronic; serves on advisory boards for Medtronic and Boston Scientific; and has equity in Transmural Systems. Dr Eng is on the Speakers Bureau for Edwards Lifesciences and Medtronic. Dr Foerst is a proctor for Edwards Lifesciences and Medtronic. Dr Paone is proctor for Edwards Lifesciences and has equity in Medtronic. Dr Lederman is the principal investigator on a cooperative research and development agreement between the National Institutes of Health and Edwards Lifesciences for transcatheter modification of the mitral valve. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


References
-
- Guerrero M, Vemulapalli S, Xiang Q et al. Thirty-Day Outcomes of Transcatheter Mitral Valve Replacement for Degenerated Mitral Bioprostheses (Valve-in-Valve), Failed Surgical Rings (Valve-in-Ring), and Native Valve With Severe Mitral Annular Calcification (Valve-in-Mitral Annular Calcification) in the United States: Data From the Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv 2020;13:e008425. - PubMed
-
- Forrestal BJ, Khan JM, Torguson R et al. Reasons for Screen Failure for Transcatheter Mitral Valve Repair and Replacement. Am J Cardiol 2021;148:130–137. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

