Safety, efficacy, and quality of life outcomes of pulsed field ablation in Japanese patients with atrial fibrillation: results from the PULSED AF trial
- PMID: 39243306
- PMCID: PMC11832727
- DOI: 10.1007/s10840-024-01912-w
Safety, efficacy, and quality of life outcomes of pulsed field ablation in Japanese patients with atrial fibrillation: results from the PULSED AF trial
Abstract
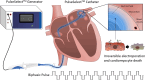
Background: Pulsed field ablation (PFA), a novel treatment for atrial fibrillation (AF), has yet to be evaluated in a Japanese cohort.
Methods: In this sub-analysis of the PULSED AF trial, 12-month outcomes of paroxysmal AF (PAF) and persistent AF (PsAF) patients treated with PFA in four Japan centers were assessed. After a 90-day blanking period, primary efficacy was determined via freedom from a composite endpoint of acute procedural failure, arrhythmia recurrence, or antiarrhythmic drug escalation over 1 year. Patient improvement was evaluated via two quality of life (QoL) surveys (AFEQT and EQ-5D) at baseline and 12 months.
Results: The analysis included 32 patients, 16 PAF and 16 PsAF, with PAF patients averaging 61.1 ± 10.6 years and PsAF patients averaging 62.8 ± 11.5 years of age. Females made up 31% of PAF and 25% of PsAF cohorts. Acute pulmonary vein isolation was achieved in 100% of both cohorts. The primary efficacy success rate at 12 months was 75.0% for PAF and 56.3% for PsAF patients. No primary safety events occurred. The mean AFEQT score significantly increased for both PAF (25.9 points, p < 0.0001) and PsAF (13.2 points, p = 0.0002) patients, while the EQ-5D-5L score improved significantly for PAF (0.12 points, p = 0.048) patients but not for PsAF (0.04 points, p = 0.08) patients.
Conclusions: Similar to outcomes in the global cohort, ablation with the PulseSelect™ PFA catheter was efficient, effective, and safe in a Japanese population, resulting in improved QoL for PAF and PsAF patients.
Clinical trial registration: ClinicalTrials.gov Identifier: NCT04198701.
Keywords: Atrial fibrillation; Catheter ablation; Japan; Pulsed field ablation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Data collection adhered to the principles outlined in the Declaration of Helsinki and Good Clinical Practices. The study was approved by local institutional review boards and ethics committees at each center. Informed consent: Patients provided written informed consent prior to participation in the study. Conflicts of interest: J Cerkvenik and Dr. JM Selma are employees of Medtronic. Dr. Miyazaki received endowed affiliations and lecture fees from Medtronic. Dr. Tada received research grants from Abbott Medical Japan LLC, Nippon Boehringer lngelheim Co., Ltd, DAIICHI SANKYO COMPANY, Ltd., Eli Lilly Japan K.K., Otsuka Pharmaceutical Co., Ltd., and Marubun Tusyo K.K. Dr. Tada also received honoraria (lecture fees, presentations, or speakers’ bureaus) from DAIICHI SANKYO COMPANY, Ltd., BIOTRONIK Japan, Inc., Bristol Myers Squibb, Boston Scientific Japan K.K., Novartis Pharma K.K., and Medtronic Japan Co., Ltd. Dr. Tomita received research funding from Boehringer Ingelheim, Bayer, Daiichi-Sankyo, Boston Scientific Japan Co., Ltd., Abbott Medical Japan LLC., Biotronik Japan Co., Ltd. and speakers’ bureau/honorarium from Boehringer Ingelheim, Bayer, Daiichi-Sankyo, and Bristol-Myers Squibb. Dr. Tomita is a concurrent professor, and Drs. Kimura and Itoh are an associate professor of an endowed department supported by Medtronic Japan Co., Ltd., Japan Lifeline Co., Ltd, and Fukuda Denshi Kita-Tohoku Hanbai Co., Ltd. Dr. Kimura received honorariums from Medtronic, Boston Scientific, Abbott, Toray, and Johnson & Johnson, and has affiliation with an endowed department supported by Medtronic, Fukuda Denshi, and Japan Lifeline. Dr. Taihei Ito received honorariums from Medtronic, Boston Scientific, Abbott, Toray, and Johnson & Johnson and has affiliation with an endowed department supported by Medtronic, Fukuda Denshi, and Japan Lifeline. Dr. Verma, received grants and/or consultation funds from Medtronic, Inc, Biosense Webster (BW), Bayer, Medlumics, Adagio Medical, and Boston Scientific.
Figures


References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. 10.1093/eurheartj/ehaa612. - PubMed
-
- Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3(1):32–8. 10.1161/CIRCEP.109.859116. - PubMed
-
- Yarmush ML, Golberg A, Sersa G, Kotnik T, Miklavcic D. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 2014;16:295–320. 10.1146/annurev-bioeng-071813-104622. - PubMed
Publication types
MeSH terms
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical

