The Cost Effectiveness of Adjunctive Treatments for Proton Pump Inhibitor-Refractory Gastroesophageal Reflux Disease
- PMID: 39243348
- PMCID: PMC11455727
- DOI: 10.1007/s40261-024-01387-7
The Cost Effectiveness of Adjunctive Treatments for Proton Pump Inhibitor-Refractory Gastroesophageal Reflux Disease
Abstract
Background and objective: Half of patients with gastroesophageal reflux disease (GERD) experience persistent symptoms while on proton pump inhibitors (PPIs), thus driving efforts to develop novel adjunctive therapies for PPI-refractory GERD. An economic analysis was performed to establish at what cost and efficacy such potential medications are likely to become cost effective in clinical practice.
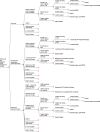
Methods: A Markov decision model was used to examine a hypothetical cohort of patients being evaluated for PPI-refractory GERD in the USA. The model compared 3 strategies: (1) usual care (i.e., upfront diagnostic testing with upper endoscopy ± ambulatory pH testing); (2) use of a PPI-adjunctive therapy after positive ambulatory pH testing; and (3) empiric use of a PPI-adjunctive therapy (i.e., diagnostic testing only after failing empiric treatment). The primary outcome was incremental cost per quality-adjusted life year (QALY) gained (third-party payer perspective) over a 10-year time horizon using a willingness to pay threshold of $100,000/QALY.
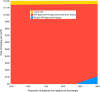
Results: In two-way sensitivity analyses varying the cost and effectiveness of the PPI-adjunctive therapy, most combinations revealed that use of the medication after positive pH testing was the most cost-effective approach. Empiric treatment was the preferred strategy only when the therapy was highly efficacious (≥ 87.5% response rate) and low cost (≤ $109/month). Use of PPI-adjunctive treatments were not cost effective when the cost exceeded $1150/month.
Conclusion: Use of PPI-adjunctive therapies in those with persistent GERD symptoms may become cost effective when guided by ambulatory pH tests. These data can guide investigators, industry, and payers as they develop, validate, and price new treatments for PPI-refractory GERD.
© 2024. The Author(s).
Conflict of interest statement
Ulysses S. Rosas: No relevant disclosures; Christopher V. Almario, MD, MSHPM: Consulted for Exact Sciences, Greenspace Labs, Owlstone Medical, and Salix Pharmaceuticals; grants to his institution from Freenome; stock options in My Total Health; Kyung-Sang Yu, MD, PhD, MBA: No relevant disclosures; Brennan M.R. Spiegel, MD, MSHS: Consulted for Ardelyx, Exact Sciences, Ferring; leadership roles in the American College of Gastroenterology (Governor for Southern California); research grants to his institution from Abbvie, Amgen, Ironwood, Salix/Bausch, Takeda; patents for My Nutrition Health, digital manometry, AbStats sensor; co-founder of VRx Health.
Figures


References
-
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101(8):1900–1920 (quiz 1943). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

