Proposed Denosumab Biosimilar SB16 vs Reference Denosumab in Postmenopausal Osteoporosis: Phase 3 Results Up to Month 12
- PMID: 39243386
- PMCID: PMC12086410
- DOI: 10.1210/clinem/dgae611
Proposed Denosumab Biosimilar SB16 vs Reference Denosumab in Postmenopausal Osteoporosis: Phase 3 Results Up to Month 12
Abstract
Context: SB16 is a proposed biosimilar to reference denosumab (DEN; brand name: Prolia).
Objective: This phase 3 randomized, double-blind, multicenter study evaluated the biosimilarity of SB16 to DEN in women with postmenopausal osteoporosis (NCT04664959).
Design: The study included 457 postmenopausal osteoporosis patients who had a lumbar spine or total hip T-score between -2.5 and -4. Patients were randomized in a 1:1 ratio to receive either 60 mg of SB16 or DEN subcutaneously at month 0 and month 6. At month 12, patients were rerandomized to continue with the assigned treatment or switch from DEN to SB16 up to month 18. This report includes results up to month 12.
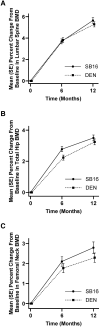
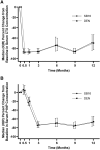
Methods: The primary endpoint was the percent change from baseline in lumbar spine bone mineral density (BMD) at month 12. Secondary endpoints including the percent change from baseline in BMD of the lumbar spine (except for month 12), total hip, and femoral neck; pharmacokinetic, pharmacodynamic (serum C-telopeptide of type I collagen, and procollagen type I N-terminal propeptide), safety, and immunogenicity profiles were measured up to month 12.
Results: The least-squares mean differences in percent change from baseline in lumbar spine BMD at month 12 were 0.33% (90% CI, -0.25 to 0.91) in the full analysis set and 0.39% (95% CI, -0.36 to 1.13) in the per-protocol set; both within the predefined equivalence margin. The secondary endpoints were comparable between the 2 treatment groups.
Conclusion: The reported efficacy, pharmacokinetic, pharmacodynamic, safety, and immunogenicity data support the biosimilarity of SB16 to DEN.
Keywords: clinical trials; menopause; metabolic bone disease; osteoporosis.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Sarafrazi N, Wambogo EA, Shepherd JA. Osteoporosis or low bone mass in older adults: United States, 2017-2018. NCHS Data Brief. 2021;(405):1‐8. - PubMed
-
- European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP) . Guideline on the Evaluation of Medicinal Products in the Treatment of Primary Osteoporosis- Rev. 2. 2006. Accessed July 22, 2024. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-ev...
-
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595‐1622. - PubMed
-
- European Medicines Agency (EMA) . Summary of Product Characteristics of Prolia. September 2023. Accessed July 22, 2024. https://www.ema.europa.eu/en/documents/product-information/prolia-epar-p...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

