Sex-dependent effects in the aged melanoma tumor microenvironment influence invasion and resistance to targeted therapy
- PMID: 39243764
- PMCID: PMC11580838
- DOI: 10.1016/j.cell.2024.08.013
Sex-dependent effects in the aged melanoma tumor microenvironment influence invasion and resistance to targeted therapy
Abstract
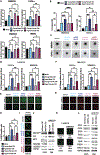
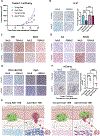
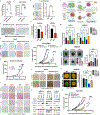
There is documented sex disparity in cutaneous melanoma incidence and mortality, increasing disproportionately with age and in the male sex. However, the underlying mechanisms remain unclear. While biological sex differences and inherent immune response variability have been assessed in tumor cells, the role of the tumor-surrounding microenvironment, contextually in aging, has been overlooked. Here, we show that skin fibroblasts undergo age-mediated, sex-dependent changes in their proliferation, senescence, ROS levels, and stress response. We find that aged male fibroblasts selectively drive an invasive, therapy-resistant phenotype in melanoma cells and promote metastasis in aged male mice by increasing AXL expression. Intrinsic aging in male fibroblasts mediated by EZH2 decline increases BMP2 secretion, which in turn drives the slower-cycling, highly invasive, and therapy-resistant melanoma cell phenotype, characteristic of the aged male TME. Inhibition of BMP2 activity blocks the emergence of invasive phenotypes and sensitizes melanoma cells to BRAF/MEK inhibition.
Keywords: DNA damage; aging; epigenetics; fibroblast; melanoma; metastasis; senescence; sex dimorphism; sex disparity; therapy resistance; tumor microenvironment.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.T.W. is on the board of reGAIN Therapeutics and the Melanoma Research Foundation. E.M.J. reports other support from Abmeta, personal fees from Genocea, personal fees from Achilles, personal fees from DragonFly, personal fees from Candel Therapeutics, other support from the Parker Institute, grants and other support from Lustgarten, personal fees from Carta, grants and other support from Genentech, grants and other support from AstraZeneca, personal fees from NextCure, and grants and other support from Break Through Cancer outside of the submitted work. D.J.Z. reports grant funding (paid to Johns Hopkins University) from Roche/Genentech. Y.C. and M.E.F. are affiliated with the Cancer Signaling and Microenvironment program, FoxChase Cancer Center, Philadelphia, PA, USA.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

