Divergent sensory pathways of sneezing and coughing
- PMID: 39243765
- PMCID: PMC11622829
- DOI: 10.1016/j.cell.2024.08.009
Divergent sensory pathways of sneezing and coughing
Abstract
Sneezing and coughing are primary symptoms of many respiratory viral infections and allergies. It is generally assumed that sneezing and coughing involve common sensory receptors and molecular neurotransmission mechanisms. Here, we show that the nasal mucosa is innervated by several discrete populations of sensory neurons, but only one population (MrgprC11+MrgprA3-) mediates sneezing responses to a multitude of nasal irritants, allergens, and viruses. Although this population also innervates the trachea, it does not mediate coughing, as revealed by our newly established cough model. Instead, a distinct sensory population (somatostatin [SST+]) mediates coughing but not sneezing, unraveling an unforeseen sensory difference between sneezing and coughing. At the circuit level, sneeze and cough signals are transmitted and modulated by divergent neuropathways. Together, our study reveals the difference in sensory receptors and neurotransmission/modulation mechanisms between sneezing and coughing, offering neuronal drug targets for symptom management in respiratory viral infections and allergies.
Keywords: allergy; coughing; respiratory viral infection; sensory neurons; sneezing.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.J.H. has noncompeting financial interests unrelated to this work and is the Founder of NuPeak Therapeutics Inc.
Figures

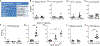


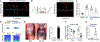
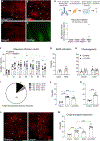
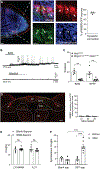
Similar articles
-
Sneezing reflex is mediated by a peptidergic pathway from nose to brainstem.Cell. 2021 Jul 8;184(14):3762-3773.e10. doi: 10.1016/j.cell.2021.05.017. Epub 2021 Jun 15. Cell. 2021. PMID: 34133943 Free PMC article.
-
Upper airway motor outputs during sneezing and coughing in decerebrate cats.Neurosci Res. 1998 Oct;32(2):131-5. doi: 10.1016/s0168-0102(98)00075-3. Neurosci Res. 1998. PMID: 9858020
-
Interactions of mechanically induced coughing and sneezing in cat.Respir Physiol Neurobiol. 2015 Jan 1;205:21-7. doi: 10.1016/j.resp.2014.09.011. Epub 2014 Sep 28. Respir Physiol Neurobiol. 2015. PMID: 25262583
-
Upper Airway Cough Syndrome in Pathogenesis of Chronic Cough.Physiol Res. 2020 Mar 27;69(Suppl 1):S35-S42. doi: 10.33549/physiolres.934400. Physiol Res. 2020. PMID: 32228010 Free PMC article. Review.
-
Sorry, I am sneezing and coughing but I do not have COVID-19.Brain Behav Immun. 2022 Mar;101:57-58. doi: 10.1016/j.bbi.2021.12.018. Epub 2021 Dec 27. Brain Behav Immun. 2022. PMID: 34968716 Free PMC article. Review. No abstract available.
Cited by
-
The sneeze reflex in physiological and pathological states: a mini review.Front Neurosci. 2025 May 9;19:1598027. doi: 10.3389/fnins.2025.1598027. eCollection 2025. Front Neurosci. 2025. PMID: 40415887 Free PMC article. Review.
-
An interorgan neuroimmune circuit promotes visceral hypersensitivity.Res Sq [Preprint]. 2025 Mar 17:rs.3.rs-6221928. doi: 10.21203/rs.3.rs-6221928/v1. Res Sq. 2025. PMID: 40166016 Free PMC article. Preprint.
-
Sensory neurons on guard: roles in pathogen defense and host immunity.Curr Opin Immunol. 2025 Apr;93:102541. doi: 10.1016/j.coi.2025.102541. Epub 2025 Feb 26. Curr Opin Immunol. 2025. PMID: 40015178 Review.
-
Cyclosporin A alleviates influenza A (H1N1) virus-induced chronic pulmonary inflammation through decreasing IFN-γ-producing T lymphocytes.Virol J. 2025 Jul 25;22(1):255. doi: 10.1186/s12985-025-02887-4. Virol J. 2025. PMID: 40713716 Free PMC article.
-
Mycobacterial Phenolic Glycolipid Triggers ATP-Mediated Neuronal P2X3 Signaling and Cough.bioRxiv [Preprint]. 2025 May 6:2025.05.01.651726. doi: 10.1101/2025.05.01.651726. bioRxiv. 2025. PMID: 40654771 Free PMC article. Preprint.
References
-
- Wallois F, Macron JM, Jounieaux V, and Duron B. (1991). Trigeminal afferences implied in the triggering or inhibition of sneezing in cats. Neurosci. Lett. 122, 145–147. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

