A phase 3, randomized, double-blind, active-controlled clinical trial to compare BAT1806/BIIB800, a tocilizumab biosimilar, with tocilizumab reference product in participants with moderate-to-severe rheumatoid arthritis with inadequate response to methotrexate: treatment period 2 analysis (week 24 to week 48)
- PMID: 39244595
- PMCID: PMC11380339
- DOI: 10.1186/s13075-024-03375-w
A phase 3, randomized, double-blind, active-controlled clinical trial to compare BAT1806/BIIB800, a tocilizumab biosimilar, with tocilizumab reference product in participants with moderate-to-severe rheumatoid arthritis with inadequate response to methotrexate: treatment period 2 analysis (week 24 to week 48)
Abstract
Background: Equivalent efficacy and comparable pharmacokinetic, immunogenicity, and safety profiles of the biosimilar BAT1806/BIIB800 and reference tocilizumab (TCZ) in participants with moderate-to-severe rheumatoid arthritis (RA) have been reported up to week 24 (treatment period [TP] 1) of the phase 3 study. Here we present results for TP2 (study weeks 24-48).
Methods: In this phase 3, multicenter, multiregional, double-blind, active-controlled, equivalence study, participants with active RA despite methotrexate were randomized (1:1:2) to intravenous administration of 8 mg/kg TCZ every 4 weeks to week 48 (TCZ group), or TCZ to week 24 followed by BAT1806/BIIB800 to week 48 (TCZ to BAT1806/BIIB800 group), or BAT1806/BIIB800 to week 48 (BAT1806/BIIB800 group). Efficacy in TP2 was evaluated using American College of Rheumatology (ACR) response criteria (ACR20/50/70) and change from baseline in Disease Activity Score on 28 joints (DAS28). Pharmacokinetics (trough levels), safety, and immunogenicity were also evaluated.
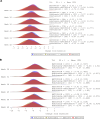
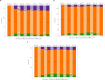
Results: Of 621 randomized participants, 577 (92.9%) completed TP1 and entered TP2 (TCZ: N = 145 [93.5%]; TCZ to BAT1806/BIIB800: N = 142 [92.2%]; BAT1806/BIIB800: N = 290 [92.9%]). Proportions of ACR20 responders were similar between treatment groups throughout TP2 (87.8%, 90.3%, and 90.4%, respectively, at week 48), as were proportions of ACR50 and ACR70 responders, and reduction in DAS28. Drug trough levels and antidrug antibody incidences were comparable between the treatment groups. Adverse events were balanced across the treatment groups and no fatal events were reported.
Conclusion: In TP2, efficacy, safety, immunogenicity, and pharmacokinetic profiles were comparable between the TCZ, TCZ to BAT1806/BIIB800, and BAT1806/BIIB800 groups.
Trial registration: NCT03830203 and EudraCT 2018-002202-31.
Keywords: Biosimilar pharmaceuticals; Rheumatoid arthritis; Tocilizumab; Treatment switching.
© 2024. The Author(s).
Conflict of interest statement
XL, SJ, SL, HL, MMia, JG, and XZ have no conflicts of interest. PL has received speaker fees from Novartis, AbbVie, UCB, MSD, and GSK; has received support for attending meetings from AbbVie, AstraZeneca, and Medac, and has received a grant for this study from Bio-Thera Solutions Ltd. LK has received speaker fees from Sandoz, Amgen, and Takeda. MS has received speaker fees from Pfizer, Orion, and Boehringer Ingelheim. XY, YZ, and QD are Bio-Thera Solutions Ltd employees and might hold stock, stock options, or both in Bio-Thera Solutions Ltd. MMit, JA, and MFR are Biogen employees and may hold stock, stock options, or both in Biogen.
Figures

References
-
- Center for Drug Evaluation of the National Medical Products Administration. Technical guidelines for the development and evaluation of biosimilar drugs (in Chinese). [Internet]. 2015. https://www.cde.org.cn/zdyz/domesticinfopage?zdyzIdCODE=f044cdf4b7d7286a.... Accessed January 12, 2023.
-
- European Medicines Agency. Guideline on similar biological medicinal products [Internet]. 2014. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-si.... Accessed August 16, 2022.
-
- US Food and Drug Administration. Development of therapeutic protein biosimilars: comparative analytical assessment and other quality-related considerations [Internet]. 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Accessed August 16, 2022.
-
- Leng X, Leszczynski P, Jeka S, Liu SY, Liu H, Miakisz M, et al. Comparing tocilizumab biosimilar BAT1806/BIIB800 with reference tocilizumab in patients with moderate-to-severe rheumatoid arthritis with an inadequate response to methotrexate: a phase 3, randomised, multicentre, double-blind, active-controlled clinical trial. Lancet Rheumatol. 2024;6(1):e40–50. 10.1016/S2665-9913(23)00237-0 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

