Artificial selection improves pollutant degradation by bacterial communities
- PMID: 39244615
- PMCID: PMC11380672
- DOI: 10.1038/s41467-024-52190-z
Artificial selection improves pollutant degradation by bacterial communities
Abstract
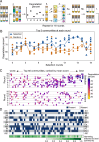
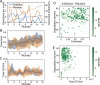
Artificial selection is a promising way to improve microbial community functions, but previous experiments have only shown moderate success. Here, we experimentally evaluate a new method that was inspired by genetic algorithms to artificially select small bacterial communities of known species composition based on their degradation of an industrial pollutant. Starting from 29 randomly generated four-species communities, we repeatedly grew communities for four days, selected the 10 best-degrading communities, and rearranged them into 29 new communities composed of four species of equal ratios whose species compositions resembled those of the most successful communities from the previous round. The best community after 18 such rounds of selection degraded the pollutant better than the best community in the first round. It featured member species that degrade well, species that degrade badly alone but improve community degradation, and free-rider species that did not contribute to community degradation. Most species in the evolved communities did not differ significantly from their ancestors in their phenotype, suggesting that genetic evolution plays a small role at this time scale. These experiments show that artificial selection on microbial communities can work in principle, and inform on how to improve future experiments.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Connors, B. M. et al. Model-guided design of the diversity of a synthetic human gut community. bioRxivhttps://www.biorxiv.org/content/10.1101/2022.03.14.484355v1 (2022). - DOI
Publication types
MeSH terms
Substances
Grants and funding
- 715097/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 715097/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 715097/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 715097/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 715097/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
LinkOut - more resources
Full Text Sources

