Intermittent ozone inhalation during house dust mite-induced sensitization primes for adverse asthma phenotype
- PMID: 39244793
- PMCID: PMC11407077
- DOI: 10.1016/j.redox.2024.103330
Intermittent ozone inhalation during house dust mite-induced sensitization primes for adverse asthma phenotype
Abstract
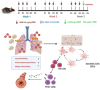
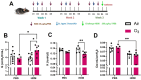
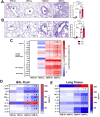
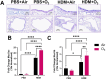
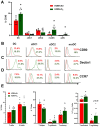
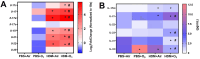
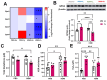
The ability of air pollution to induce acute exacerbation of asthma is well documented. However, the ability of ozone (O3), the most reactive gaseous component of air pollution, to function as a modulator during sensitization is not well established. C57BL/6 J male mice were intranasally sensitized to house dust mite (HDM) (40 μg/kg) for 3 weeks on alternate days in parallel with once-a-week O3 exposure (1 ppm). Mice were euthanized 24 h following the last HDM challenge. Lung lavage, histology, lung function (both forced oscillation and forced expiration-based), immune cell profiling, inflammation (pulmonary and systemic), and immunoglobulin production were assessed. Compared to HDM alone, HDM + O3 leads to a significant increase in peribronchial inflammation (p < 0.01), perivascular inflammation (p < 0.001) and methacholine-provoked large airway hyperreactivity (p < 0.05). Serum total IgG and IgE and HDM-specific IgG1 were 3-5 times greater in HDM + O3 co-exposure compared to PBS and O3-exposed groups. An increase in activated/mature lung total and monocyte-derived dendritic cells (p < 0.05) as well as T-activated, and T memory lymphocyte subset numbers (p < 0.05) were noted in the HDM + O3 group compared to HDM alone group. Concurrent O3 inhalation and HDM sensitization also caused significantly greater (p < 0.05) lung tissue interleukin-17 pathway gene expression and mediator levels in the serum. Redox imbalance was manifested by impaired lung antioxidant defense and increased oxidants. O3 inhalation during allergic sensitization coalesces in generating a significantly worse TH17 asthmatic phenotype.
Keywords: Airway hyperresponsiveness; Inflammation; Lung; Lung function; Ozone; T(H)17.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Buteau S., Doucet M., Tetreault L.F., Gamache P., Fournier M., Brand A., Kosatsky T., Smargiassi A. A population-based birth cohort study of the association between childhood-onset asthma and exposure to industrial air pollutant emissions. Environ. Int. 2018;121(Pt 1):23–30. doi: 10.1016/j.envint.2018.08.040. - DOI - PubMed
-
- Tuazon J.A., Kilburg-Basnyat B., Oldfield L.M., Wiscovitch-Russo R., Dunigan-Russell K., Fedulov A.V., Oestreich K.J., Gowdy K.M. Emerging insights into the impact of air pollution on immune-mediated asthma pathogenesis. Curr. Allergy Asthma Rep. 2022;22(7):77–92. doi: 10.1007/s11882-022-01034-1. - DOI - PMC - PubMed
-
- Rosenquist N.A., Metcalf W.J., Ryu S.Y., Rutledge A., Coppes M.J., Grzymski J.J., Strickland M.J., Darrow L.A. Acute associations between PM2.5 and ozone concentrations and asthma exacerbations among patients with and without allergic comorbidities. J. Expo. Sci. Environ. Epidemiol. 2020;30(5):795–804. doi: 10.1038/s41370-020-0213-7. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

