Microglial morphological/inflammatory phenotypes and endocannabinoid signaling in a preclinical model of periodontitis and depression
- PMID: 39245706
- PMCID: PMC11382403
- DOI: 10.1186/s12974-024-03213-5
Microglial morphological/inflammatory phenotypes and endocannabinoid signaling in a preclinical model of periodontitis and depression
Abstract
Background: Depression is a chronic psychiatric disease of multifactorial etiology, and its pathophysiology is not fully understood. Stress and other chronic inflammatory pathologies are shared risk factors for psychiatric diseases, and comorbidities are features of major depression. Epidemiological evidence suggests that periodontitis, as a source of low-grade chronic systemic inflammation, may be associated with depression, but the underlying mechanisms are not well understood.
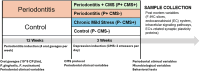
Methods: Periodontitis (P) was induced in Wistar: Han rats through oral gavage with the pathogenic bacteria Porphyromonas gingivalis and Fusobacterium nucleatum for 12 weeks, followed by 3 weeks of chronic mild stress (CMS) to induce depressive-like behavior. The following four groups were established (n = 12 rats/group): periodontitis and CMS (P + CMS+), periodontitis without CMS, CMS without periodontitis, and control. The morphology and inflammatory phenotype of microglia in the frontal cortex (FC) were studied using immunofluorescence and bioinformatics tools. The endocannabinoid (EC) signaling and proteins related to synaptic plasticity were analyzed in FC samples using biochemical and immunohistochemical techniques.
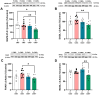
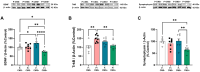
Results: Ultrastructural and fractal analyses of FC revealed a significant increase in the complexity and heterogeneity of Iba1 + parenchymal microglia in the combined experimental model (P + CMS+) and increased expression of the proinflammatory marker inducible nitric oxide synthase (iNOS), while there were no changes in the expression of cannabinoid receptor 2 (CB2). In the FC protein extracts of the P + CMS + animals, there was a decrease in the levels of the EC metabolic enzymes N-acyl phosphatidylethanolamine-specific phospholipase D (NAPE-PLD), diacylglycerol lipase (DAGL), and monoacylglycerol lipase (MAGL) compared to those in the controls, which extended to protein expression in neurons and in FC extracts of cannabinoid receptor 1 (CB1) and to the intracellular signaling molecules phosphatidylinositol-3-kinase (PI3K), protein kinase B (Akt) and extracellular signal-regulated kinase 1/2 (ERK1/2). The protein levels of brain-derived neurotrophic factor (BDNF) and synaptophysin were also lower in P + CMS + animals than in controls.
Conclusions: The combined effects on microglial morphology and inflammatory phenotype, the EC signaling, and proteins related to synaptic plasticity in P + CMS + animals may represent relevant mechanisms explaining the association between periodontitis and depression. These findings highlight potential therapeutic targets that warrant further investigation.
Keywords: Depression; Endocannabinoid signaling; Microglia; Periodontitis; Synaptic plasticity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- GBD: Global Burden of Disease Study 2019. (GBD 2019) Results tool. Seattle, United States of America: Institute for Health Metrics and Evaluation (IHME); 2021.
-
- World Health A. Global burden of mental disorders and the need for a comprehensive, coordinated response from health and social sectors at the country level: report by the Secretariat. Geneva: World Health Organization; 2012.
-
- Yuan K, Zheng YB, Wang YJ, Sun YK, Gong YM, Huang YT, Chen X, Liu XX, Zhong Y, Su SZ, et al. A systematic review and meta-analysis on prevalence of and risk factors associated with depression, anxiety and insomnia in infectious diseases, including COVID-19: a call to action. Mol Psychiatry. 2022;27:3214–22. 10.1038/s41380-022-01638-z - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

