Glucokinase (GCK) in diabetes: from molecular mechanisms to disease pathogenesis
- PMID: 39245718
- PMCID: PMC11382428
- DOI: 10.1186/s11658-024-00640-3
Glucokinase (GCK) in diabetes: from molecular mechanisms to disease pathogenesis
Abstract
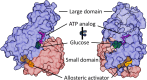
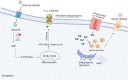
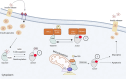
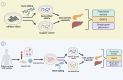
Glucokinase (GCK), a key enzyme in glucose metabolism, plays a central role in glucose sensing and insulin secretion in pancreatic β-cells, as well as glycogen synthesis in the liver. Mutations in the GCK gene have been associated with various monogenic diabetes (MD) disorders, including permanent neonatal diabetes mellitus (PNDM) and maturity-onset diabetes of the young (MODY), highlighting its importance in maintaining glucose homeostasis. Additionally, GCK gain-of-function mutations lead to a rare congenital form of hyperinsulinism known as hyperinsulinemic hypoglycemia (HH), characterized by increased enzymatic activity and increased glucose sensitivity in pancreatic β-cells. This review offers a comprehensive exploration of the critical role played by the GCK gene in diabetes development, shedding light on its expression patterns, regulatory mechanisms, and diverse forms of associated monogenic disorders. Structural and mechanistic insights into GCK's involvement in glucose metabolism are discussed, emphasizing its significance in insulin secretion and glycogen synthesis. Animal models have provided valuable insights into the physiological consequences of GCK mutations, although challenges remain in accurately recapitulating human disease phenotypes. In addition, the potential of human pluripotent stem cell (hPSC) technology in overcoming current model limitations is discussed, offering a promising avenue for studying GCK-related diseases at the molecular level. Ultimately, a deeper understanding of GCK's multifaceted role in glucose metabolism and its dysregulation in disease states holds implications for developing targeted therapeutic interventions for diabetes and related disorders.
Keywords: Beta cells; Diabetes; Glucokinase; Glucose; Insulin; Liver; Mutations; Pancreas; Stem cells.
© 2024. The Author(s).
Conflict of interest statement
A.K.K.T. is a co-founder and shareholder of BetaLife Pte Ltd but is not employed by BetaLife Pte Ltd. All other authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

