Gene and cell therapy of human genetic diseases: Recent advances and future directions
- PMID: 39245805
- PMCID: PMC11381193
- DOI: 10.1111/jcmm.70056
Gene and cell therapy of human genetic diseases: Recent advances and future directions
Abstract
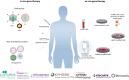
Disruptions in normal development and the emergence of health conditions often result from the malfunction of vital genes in the human body. Decades of scientific research have focused on techniques to modify or substitute defective genes with healthy alternatives, marking a new era in disease treatment, prevention and cure. Recent strides in science and technology have reshaped our understanding of disorders, medication development and treatment recommendations, with human gene and cell therapy at the forefront of this transformative shift. Its primary objective is the modification of genes or adjustment of cell behaviour for therapeutic purposes. In this review, we focus on the latest advances in gene and cell therapy for treating human genetic diseases, with a particular emphasis on FDA and EMA-approved therapies and the evolving landscape of genome editing. We examine the current state of innovative gene editing technologies, particularly the CRISPR-Cas systems. As we explore the progress, ethical considerations and prospects of these innovations, we gain insight into their potential to revolutionize the treatment of genetic diseases, along with a discussion of the challenges associated with their regulatory pathways. This review traces the origins and evolution of these therapies, from conceptual ideas to practical clinical applications, marking a significant milestone in the field of medical science.
Keywords: CRISPR/Cas9; cell therapy; gene editing; gene therapy; viral vectors.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures

References
-
- Gene therapy clinical trials worldwide . 2023; https://a873679.fmphost.com/fmi/webd/GTCT.
-
- Nilay KA, Damke S. A review on gene therapy. J Pharm Res Int. 2021;33(60B):635‐645.
-
- Handorf AM, Sollinger HW, Alam T. Insulin gene therapy for type 1 diabetes mellitus. Exp Clin Transplant. 2015;13:37‐45. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

