Novel Endogenous Engineering Platform for Robust Loading and Delivery of Functional mRNA by Extracellular Vesicles
- PMID: 39246205
- PMCID: PMC11558116
- DOI: 10.1002/advs.202407619
Novel Endogenous Engineering Platform for Robust Loading and Delivery of Functional mRNA by Extracellular Vesicles
Abstract
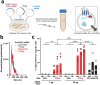
Messenger RNA (mRNA) has emerged as an attractive therapeutic molecule for a plethora of clinical applications. For in vivo functionality, mRNA therapeutics require encapsulation into effective, stable, and safe delivery systems to protect the cargo from degradation and reduce immunogenicity. Here, a bioengineering platform for efficient mRNA loading and functional delivery using bionormal nanoparticles, extracellular vesicles (EVs), is established by expressing a highly specific RNA-binding domain fused to CD63 in EV producer cells stably expressing the target mRNA. The additional combination with a fusogenic endosomal escape moiety, Vesicular Stomatitis Virus Glycoprotein, enables functional mRNA delivery in vivo at doses substantially lower than currently used clinically with synthetic lipid-based nanoparticles. Importantly, the application of EVs loaded with effective cancer immunotherapy proves highly effective in an aggressive melanoma mouse model. This technology addresses substantial drawbacks currently associated with EV-based nucleic acid delivery systems and is a leap forward to clinical EV applications.
Keywords: bioengineering; cancer immunotherapy; drug delivery; extracellular vesicles; nanotechnology; nucleic acid therapeutics.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare the following competing interests: S.E.A. is a co‐founder, consultant, and stakeholder of Evox Therapeutics Ltd. J.N., D.G., O.W., and A.G. are consultants and/or stakeholders of Evox Therapeutics Ltd. V.C.L., M.D.L., L.E., J.H., and T.S. are current or former employees of Evox Therapeutics Ltd. This work is protected by patent families WO2019092145 and WO2020225392 owned by Evox Therapeutics Ltd. Patent information WO2019092145: applicant – Evox Therapeutics Ltd; inventors – J.N., D.G., L.E., and J.H.; international application number – PCT/EP2018/08 0681. Patent information WO2020225392: applicant – Evox Therapeutics Ltd; inventors – V.C.L, A.Z., X.L., M.D.L., and L.E.; international application number – PCT/EP2020/06 2791. D.M., G.C., O.E., N.K., Z.N., G.Z., H.Z., and S.R. declare no conflict of interest.
Figures



References
-
- Weide B., Pascolo S., Scheel B., Derhovanessian E., Pflugfelder A., Eigentler T. K., Pawelec G., Hoerr I., Rammensee H. G., Garbe C., J. Immunother. 2009, 32, 498. - PubMed
-
- Sahin U., Karikó K., Türeci Ö., Nat. Rev. Drug Discovery 2014, 13, 759. - PubMed
-
- Vallazza B., Petri S., Poleganov M. A., Eberle F., Kuhn A. N., Sahin U., Wiley Interdiscip. Rev. RNA 2015, 6, 471. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
