Recent Advances in the Application of 2-Aminobenzothiazole to the Multicomponent Synthesis of Heterocycles
- PMID: 39246248
- PMCID: PMC11564876
- DOI: 10.1002/open.202400185
Recent Advances in the Application of 2-Aminobenzothiazole to the Multicomponent Synthesis of Heterocycles
Abstract
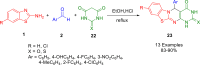
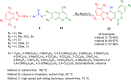
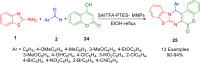
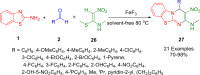
Heterocycles are a vital class of compounds in numerous fields, including drug discovery, agriculture, and materials science. Efficient methods for the synthesis of heterocycles remain critical for meeting the demands of these industries. Recent advances in multicomponent reactions (MCRs) utilizing 2-aminobenzothiazole (ABT) have shown promising results for the formation of heterocycles. The versatility of 2-aminobenzothiazole in this context has enabled the rapid and efficient construction of diverse heterocyclic structures. Various synthetic methodologies and reactions involving 2-aminobenzothiazole are discussed, highlighting its importance as a valuable building block in the synthesis of complex heterocycles. The potential applications of these heterocycles in drug discovery and material science are also explored. Overall, this review provides a comprehensive overview of the current state of research in the field and offers insights into the future directions of this promising area of study. We highlight the potential of ABT as a versatile and sustainable starting material in heterocyclic synthesis via MCRs, with significant implications for the chemical industry.
Keywords: 2-aminobenzothiazole; heterocycles; multicomponent reactions; synthesis.
© 2024 The Authors. ChemistryOpen published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




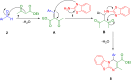







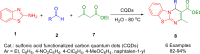
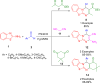
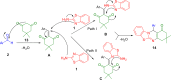
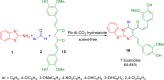
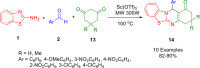

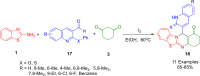
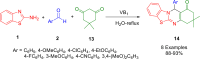
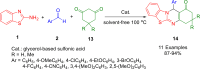
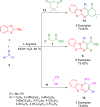
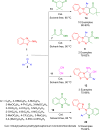
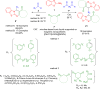


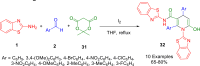

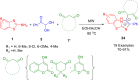
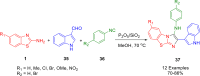
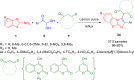
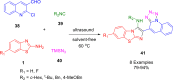
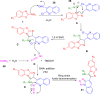
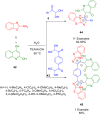
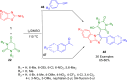
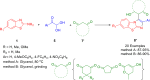
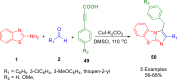
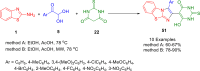
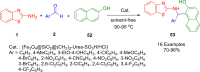
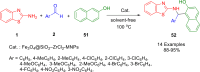

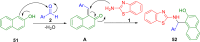

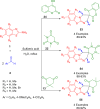
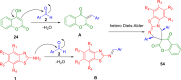
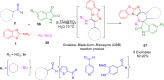
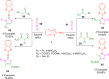

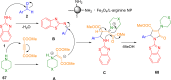

References
-
- Jangale A. D., Dalal D. S., Synth. Commun. 2017, 47, 2139.
-
- Taylor A. P., Robinson R. P., Fobian Y. M., Blakemore D. C., Jones L. H., Fadeyi O., Org. Biomol. Chem. 2016, 14, 6611. - PubMed
-
- Zhang T. Y., in Advances in Heterocyclic Chemistry, Academic Press, 2017, 121, 1.
-
- Jampilek J., Molecules 2019, 24, 3839.
Publication types
LinkOut - more resources
Full Text Sources

