Nitrogen-to-functionalized carbon atom transmutation of pyridine
- PMID: 39246332
- PMCID: PMC11372446
- DOI: 10.1039/d4sc04413d
Nitrogen-to-functionalized carbon atom transmutation of pyridine
Abstract
The targeted and selective replacement of a single atom in an aromatic system represents a powerful strategy for the rapid interconversion of molecular scaffolds. Herein, we report a pyridine-to-benzene transformation via nitrogen-to-carbon skeletal editing. This approach proceeds via a sequence of pyridine ring-opening, imine hydrolysis, olefination, electrocyclization, and aromatization to achieve the desired transmutation. The most notable features of this transformation are the ability to directly install a wide variety of versatile functional groups in the benzene scaffolding, including ester, ketone, amide, nitrile, and phosphate ester fragments, as well as the inclusion of meta-substituted pyridines which have thus far been elusive for related strategies.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

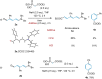

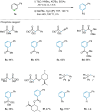
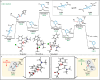
References
-
- Jurczyk J. Woo J. Kim S. F. Dherange B. D. Sarpong R. Levin M. D. Nat. Synth. 2022;1:352–364. doi: 10.1038/s44160-022-00052-1. - DOI - PMC - PubMed
- Hui C. Wang Z. Wang S. Xu C. Org. Chem. Front. 2022;9:1451–1457. doi: 10.1039/D2QO00043A. - DOI
- Joynson B. W. Ball L. T. Helv. Chim. Acta. 2023;106:e202200182. doi: 10.1002/hlca.202200182. - DOI
- Liu Z. Sivaguru P. Ning Y. Wu Y. Bi X. Chem.–Eur. J. 2023;29:e202301227. doi: 10.1002/chem.202301227. - DOI - PubMed
-
- Qiu X. Sang Y. Wu H. Xue X. S. Yan Z. Wang Y. Cheng Z. Wang X. Tan H. Song S. Zhang G. Zhang X. Houk K. N. Jiao N. Nature. 2021;597:64–69. doi: 10.1038/s41586-021-03801-y. - DOI - PubMed
- Reisenbauer J. C. Green O. Franchino A. Finkelstein P. Morandi B. Science. 2022;377:1104–1109. doi: 10.1126/science.add1383. - DOI - PubMed
- Wang J. Lu H. He Y. Jing C. Wei H. J. Am. Chem. Soc. 2022;144:22433–22439. doi: 10.1021/jacs.2c10570. - DOI - PubMed
- Bartholomew G. L. Carpaneto F. Sarpong R. J. Am. Chem. Soc. 2022;144:22309–22315. doi: 10.1021/jacs.2c10746. - DOI - PMC - PubMed
- Wang H. Shao H. Das A. Dutta S. Chan H. T. Daniliuc C. Houk K. N. Glorius F. Science. 2023;381:75–81. doi: 10.1126/science.adh9737. - DOI - PubMed
- Wright B. A. Matviitsuk A. Black M. J. Garcia-Reynaga P. Hanna L. E. Herrmann A. T. Ameriks M. K. Sarpong R. Lebold T. P. J. Am. Chem. Soc. 2023;145:10960–10966. doi: 10.1021/jacs.3c02616. - DOI - PMC - PubMed
- Joynson B. W. Cumming G. R. Ball L. T. Angew. Chem., Int. Ed. 2023;62:e202305081. doi: 10.1002/anie.202305081. - DOI - PMC - PubMed
- Woo J. Stein C. Christian A. H. Levin M. D. Nature. 2023;623:77–82. doi: 10.1038/s41586-023-06613-4. - DOI - PMC - PubMed
- Finkelstein P. Reisenbauer J. C. Botlik B. B. Green O. Florin A. Morandi B. Chem. Sci. 2023;14:2954–2959. doi: 10.1039/D2SC06952K. - DOI - PMC - PubMed
- Li H. Li N. Wu J. Yu T. Zhang R. Xu L. P. Wei H. J. Am. Chem. Soc. 2023;144:22433–22439.
- Boudry E. Bourdreux F. Marrot J. Moreau X. Ghiazza C. J. Am. Chem. Soc. 2024;146:2845–2854. doi: 10.1021/jacs.3c14467. - DOI - PubMed
- Nguyen H. M. H. Thomas D. C. Hart M. A. Steenback K. R. Levy J. N. McNally A. J. Am. Chem. Soc. 2024;146:2944–2949. doi: 10.1021/jacs.3c12445. - DOI - PMC - PubMed
- Bartholomew G. L. Kraus S. L. Karas L. J. Carpaneto F. Bennett R. Sigman M. S. Yeung C. S. Sarpong R. J. Am. Chem. Soc. 2024;146:2950–2958. doi: 10.1021/jacs.3c11515. - DOI - PMC - PubMed
- Tolchin Z. A. Smith J. M. J. Am. Chem. Soc. 2024;146:2939–2943. doi: 10.1021/jacs.3c11618. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

