Comprehensive understanding of the adverse effects associated with temozolomide: a disproportionate analysis based on the FAERS database
- PMID: 39246656
- PMCID: PMC11377320
- DOI: 10.3389/fphar.2024.1437436
Comprehensive understanding of the adverse effects associated with temozolomide: a disproportionate analysis based on the FAERS database
Abstract
Background: Temozolomide, which is the standard drug for glioma treatment, has several Adverse events (AEs) in the treatment of gliomas and other tumors that are not yet fully understood. This is due to the pharmacological nature of the alkylating agent. A significant proportion of these effects have not been systematically documented or reported.
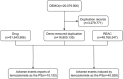
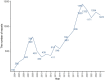
Methods: We selected data from the United States FDA Adverse Event Reporting System (FAERS) database from the first quarter of 2004 to the fourth quarter of 2023. Four algorithms were used for disproportionate analysis, with the objective of assessing the association between temozolomide and related adverse events.
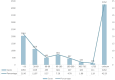
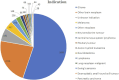
Results: In this study, 20,079,906 case reports were collected from the FAERS database, of which 15,152 adverse events related to temozolomide were reported. A total of 352 preferred terms (PTs) and 24 system organ classes (SOCs) that were significantly disproportionally related to the four algorithms were included. The SOCs included blood and lymphatic system disorders (χ2 = 18,220.09, n = 4,325); skin and subcutaneous tissue disorders (χ2 = 408.06, n = 1,347); investigations (χ2 = 639.44, n = 3,925); musculoskeletal and connective tissue disorders (χ2 = 1,317.29, n = 588); and psychiatric disorders (χ2 = 1,098.47, n = 877). PT levels were screened for adverse drug reaction signals consistent with drug inserts, such as anemia, thrombocytopenia, liver function abnormalities, nausea and vomiting, as well as rarely reported adverse drug reactions, such as aplastic anemia, myelodysplastic syndromes, electrolyte disorders, cerebral edema, and high-frequency mutations.
Conclusion: The results of our investigation demonstrated both adverse effects that had been reported and a multitude of unreported adverse effects that were serious in nature and lacked a clear cause. These novel findings suggest that more attention should be given to the clinical conditions of patients after treatment to provide a more comprehensive perspective and understanding for further clarifying the safety of temozolomide.
Keywords: FAERS; adverse effects; glioma; pharmacovigilance; real-world data analysis; temozolomide.
Copyright © 2024 Zhou, Jia, Fang, Zhu, Gong, Fan and Yin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Metformin adverse event profile: a pharmacovigilance study based on the FDA Adverse Event Reporting System (FAERS) from 2004 to 2022.Expert Rev Clin Pharmacol. 2024 Jan-Jun;17(2):189-201. doi: 10.1080/17512433.2024.2306223. Epub 2024 Jan 29. Expert Rev Clin Pharmacol. 2024. PMID: 38269492
-
Safety of triazole antifungals: a pharmacovigilance study from 2004 to 2021 based on FAERS.Ther Adv Drug Saf. 2022 Dec 16;13:20420986221143266. doi: 10.1177/20420986221143266. eCollection 2022. Ther Adv Drug Saf. 2022. PMID: 36545565 Free PMC article.
-
Pharmacovigilance analysis of orlistat adverse events based on the FDA adverse event reporting system (FAERS) database.Heliyon. 2024 Jul 18;10(14):e34837. doi: 10.1016/j.heliyon.2024.e34837. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39149028 Free PMC article.
-
Disproportionality analysis of quinolone safety in children using data from the FDA adverse event reporting system (FAERS).Front Pediatr. 2023 Jan 11;10:1069504. doi: 10.3389/fped.2022.1069504. eCollection 2022. Front Pediatr. 2023. PMID: 36714649 Free PMC article.
-
A disproportionality analysis of adverse events associated to pertuzumab in the FDA Adverse Event Reporting System (FAERS).BMC Pharmacol Toxicol. 2023 Nov 13;24(1):62. doi: 10.1186/s40360-023-00702-w. BMC Pharmacol Toxicol. 2023. PMID: 37957717 Free PMC article.
Cited by
-
Plant Alkaloids as Promising Anticancer Compounds with Blood-Brain Barrier Penetration in the Treatment of Glioblastoma: In Vitro and In Vivo Models.Molecules. 2025 Mar 31;30(7):1561. doi: 10.3390/molecules30071561. Molecules. 2025. PMID: 40286187 Free PMC article. Review.
-
Comparative safety analysis of bevacizumab and alkylating agent in glioblastoma management - What have we learned recently?Front Pharmacol. 2025 May 15;16:1595642. doi: 10.3389/fphar.2025.1595642. eCollection 2025. Front Pharmacol. 2025. PMID: 40444037 Free PMC article.
-
Neurological adverse events associated with baclofen: a pharmacovigilance study based on FDA adverse event reporting system.Front Pharmacol. 2025 May 14;16:1569602. doi: 10.3389/fphar.2025.1569602. eCollection 2025. Front Pharmacol. 2025. PMID: 40438599 Free PMC article.
References
LinkOut - more resources
Full Text Sources

