A pilot clinical study to evaluate feasibility of using single patient classifier as a prognostic test in stage II - III gastric cancer patients
- PMID: 39246705
- PMCID: PMC11377881
- DOI: 10.21147/j.issn.1000-9604.2024.04.02
A pilot clinical study to evaluate feasibility of using single patient classifier as a prognostic test in stage II - III gastric cancer patients
Abstract
Objective: Precision medicine approaches emphasize the importance of reliable prognostic tools for guiding individualized therapy decisions. In this study, we evaluated the clinical feasibility of the single patient classifier (SPC) test, a new clinical-grade prognostic assay, in stage II-III gastric cancer patients.
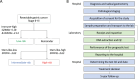
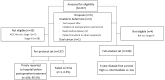
Methods: A prospective multicenter study was conducted, involving 237 patients who underwent gastrectomy between September 2019 and August 2020 across nine hospitals. The SPC test was employed to stratify patients into risk groups, and its feasibility and performance were evaluated. The primary endpoint was the proportion of the cases in which the test results were timely delivered before selecting postoperative treatment. Furthermore, 3-year disease-free survivals of risk groups were analyzed.
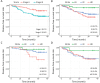
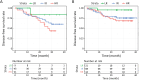
Results: The SPC test met the primary endpoint criteria. The 99.5% of SPC tests were timely delivered to hospitals before the postoperative treatment started. In a clinical setting, the median time from the specimen transfer to laboratory to the result delivery to hospital was 4 d. Furthermore, 3-year disease-free survivals were significantly different between risk groups classified with SPC tests.
Conclusions: This study highlights the SPC test's feasibility in offering crucial information timely delivered for making informed decisions regarding postoperative treatment strategies. It also provides evidence to support the implementation of a future prospective clinical trial aimed at evaluating the clinical utility of the SPC test in guiding personalized treatment decisions for gastric cancer patients.
Keywords: Prognostic test; adjuvant chemotherapy; advanced gastric cancer; feasibility; gastrectomy.
Copyright ©2024 Chinese Journal of Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
References
-
-
National Comprehensive Cancer Network. Clinical practice guidelines in oncology. Gastric cancer. Version 2. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
-
LinkOut - more resources
Full Text Sources
Miscellaneous
