Next-generation EGFR tyrosine kinase inhibitors to overcome C797S mutation in non-small cell lung cancer (2019-2024)
- PMID: 39246743
- PMCID: PMC11376191
- DOI: 10.1039/d4md00384e
Next-generation EGFR tyrosine kinase inhibitors to overcome C797S mutation in non-small cell lung cancer (2019-2024)
Abstract
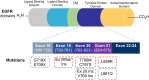
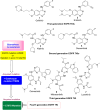
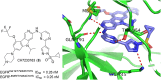
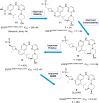
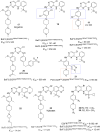
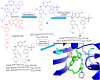
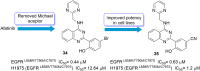
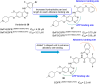
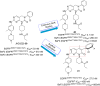
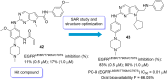
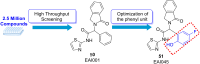
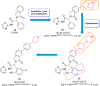
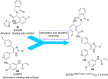
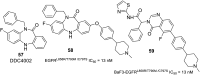
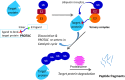
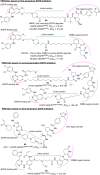
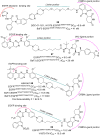
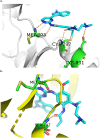
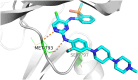
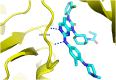
Lung cancer is a leading cause of cancer-related deaths worldwide. Non-small cell lung cancer (NSCLC) accounts for the major portion (80-85%) of all lung cancer cases. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are commonly used as the targeted therapy for EGFR-mutated NSCLC. The FDA has approved first-, second- and third-generation EGFR-TKIs as therapeutics options. Osimertinib, the third-generation irreversible EGFR-TKI, has been approved for the treatment of NSCLC patients with the EGFRT790M mutation. However, due to the EGFRC797S mutation in the kinase domain of EGFR, resistance to osimertinib is observed and that limits the long-term effectiveness of the drug. The C797S mutation is one of the major causes of drug resistance against the third-generation EGFR TKIs. The C797S mutations including EGFR double mutations (19Del/C797S or L858R/C797S) and or EGFR triple mutations (19Del/T790M/C797S or L858R/T790M/C797S) cause major resistance to the third-generation EGFR-TKIs. Therefore, the discovery and development of fourth-generation EGFR-TKIs to target triple mutant EGFR with C797S mutation is a challenging topic in medicinal chemistry research. In this review, we discuss the discovery of novel fourth-generation EGFR TKIs, medicinal chemistry approaches and the strategies to overcome the C797S mutations. In vitro activities of EGFR-TKIs (2019-2024) against mutant EGFR TK, anti-proliferative activities, structural modifications, binding modes of the inhibitors and in vivo efficacies in animal models are discussed here.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict or competing financial interest.
Figures




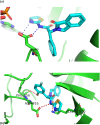
References
-
- Bray F. Laversanne M. Sung H. Ferlay J. Siegel R. L. Soerjomataram I. Jemal A. Ca-Cancer J. Clin. 2024:caac.21834. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

