In vivo liver targeted genome editing as therapeutic approach: progresses and challenges
- PMID: 39246827
- PMCID: PMC11378722
- DOI: 10.3389/fgeed.2024.1458037
In vivo liver targeted genome editing as therapeutic approach: progresses and challenges
Abstract
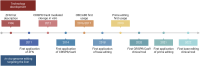
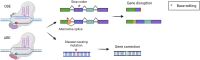
The liver is an essential organ of the body that performs several vital functions, including the metabolism of biomolecules, foreign substances, and toxins, and the production of plasma proteins, such as coagulation factors. There are hundreds of genetic disorders affecting liver functions and, for many of them, the only curative option is orthotopic liver transplantation, which nevertheless entails many risks and long-term complications. Some peculiar features of the liver, such as its large blood flow supply and the tolerogenic immune environment, make it an attractive target for in vivo gene therapy approaches. In recent years, several genome-editing tools mainly based on the clustered regularly interspaced short palindromic repeats associated protein 9 (CRISPR-Cas9) system have been successfully exploited in the context of liver-directed preclinical or clinical therapeutic applications. These include gene knock-out, knock-in, activation, interference, or base and prime editing approaches. Despite many achievements, important challenges still need to be addressed to broaden clinical applications, such as the optimization of the delivery methods, the improvement of the editing efficiency, and the risk of on-target or off-target unwanted effects and chromosomal rearrangements. In this review, we highlight the latest progress in the development of in vivo liver-targeted genome editing approaches for the treatment of genetic disorders. We describe the technological advancements that are currently under investigation, the challenges to overcome for clinical applicability, and the future perspectives of this technology.
Keywords: delivery methods; genome editing; lipid nano particle; liver; liver directed genome editing; viral vectors.
Copyright © 2024 Simoni, Barbon, Muro and Cantore.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

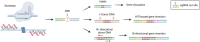
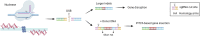




References
Publication types
LinkOut - more resources
Full Text Sources

