From mitochondria to tumor suppression: ACAT1's crucial role in gastric cancer
- PMID: 39247186
- PMCID: PMC11377227
- DOI: 10.3389/fimmu.2024.1449525
From mitochondria to tumor suppression: ACAT1's crucial role in gastric cancer
Abstract
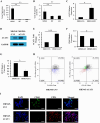
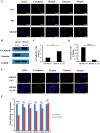
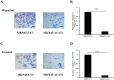
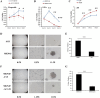
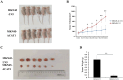
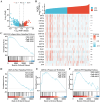
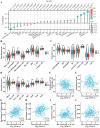
Acetyl CoA acetyltransferase 1 (ACAT1), a mitochondrial enzyme, is mainly involved in the formation and decomposition of ketones, isoleucine, and fatty acids. Previous clinical studies showed that mutations in the ACAT1 gene lead to ketoacidosis, Notably the role of ACAT1 in human cancer' pathogenesis varies depending on cancer type, and its specific role in gastric cancer remains largely unknown. In the current study, we found that the expression of ACAT1 in primary late-stage gastric cancer tumor tissues was significantly lower than in early-stage tumors. This observation was further confirmed in high-grade gastric cancer cell line MKN45. The expression of CD44 and OCT4 was decreased, while CD24 expression was increased by overexpressing ACAT1 in MKN45 gastric cancer cells. Moreover, the ability of gastric cancer cells to form colonies on soft agar was also reduced by ACAT1 overexpression. Likewise, overexpression of ACAT1 inhibited epithelial mesenchymal transition (EMT) in gastric cancer cells evidenced by increased expression of the epithelial marker E-Cadherin, decreased expression of mesenchymal marker vimentin, and decreased expression levels of SNAI 1/3. In addition, ACAT1 overexpression inhibited cell migration and invasion, improved the response to 5-Fluorouracil (5-FU) and etoposide. In contrast, inhibition of ACAT1 activity promoted the proliferation of gastric cancer cells. The xenotransplantation results in nude mice showed that overexpression of ACAT1 in gastric cancer cells inhibited tumor growth in vivo. In addition, the low expression of ACAT1 in gastric cancer was further validated by searching public databases and conducting bioinformatic analyses. Mechanistically, bioinformatic analysis found that the inhibitory effect of ACAT1 in gastric cancer may be related to the Adipocytokine Signaling Pathway, Ppar Signaling Pathway, Propanoate Metabolism and P53 Signaling Pathway. Correlation analysis indicated ACAT1 mRNA expression was correlated with immune infiltrates. Collectively, our data show that ACAT1 induces pronounced inhibitory effects on gastric cancer initiation and development, which may impact future strategies to treat this aggressive cancer.
Keywords: ACAT1; EMT; gastric cancer; mitochondrial enzyme; tumor stem cells.
Copyright © 2024 He, Li, Liu, Chang, Han, Han, Ma, Amin, Song and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

