Toxicity in the era of immune checkpoint inhibitor therapy
- PMID: 39247203
- PMCID: PMC11377343
- DOI: 10.3389/fimmu.2024.1447021
Toxicity in the era of immune checkpoint inhibitor therapy
Abstract
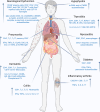
Immune checkpoint inhibitors (ICIs) reinvigorate anti-tumor immune responses by disrupting co-inhibitory immune checkpoint molecules such as programmed cell death 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4). Although ICIs have had unprecedented success and have become the standard of care for many cancers, they are often accompanied by off-target inflammation that can occur in any organ system. These immune related adverse events (irAEs) often require steroid use and/or cessation of ICI therapy, which can both lead to cancer progression. Although irAEs are common, the detailed molecular and immune mechanisms underlying their development are still elusive. To further our understanding of irAEs and develop effective treatment options, there is pressing need for preclinical models recapitulating the clinical settings. In this review, we describe current preclinical models and immune implications of ICI-induced skin toxicities, colitis, neurological and endocrine toxicities, pneumonitis, arthritis, and myocarditis along with their management.
Keywords: immune checkpoint; immune related adverse events; immunotherapy; preclinical model; treatment.
Copyright © 2024 Keam, Turner, Kugeratski, Rico, Colunga-Minutti, Poojary, Alekseev, Patel, Li, Sheshadri, Loghin, Woodman, Aaroe, Hamidi, Iyer, Palaskas, Wang and Nurieva.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Wen L, Chen Z, Ji X, Fong WP, Shao Q, Ren C, et al. Pathological complete response to immune checkpoint inhibitor in patients with colorectal cancer liver metastases harboring POLE exonuclease domain mutation. J Immunotherapy Cancer. (2022) 10:e004487. doi: 10.1136/jitc-2022-004487 - DOI - PMC - PubMed
-
- Kato K, Cho BC, Takahashi M, Okada M, Lin C-Y, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:1506–17. doi: 10.1016/S1470-2045(19)30626-6 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

