Rational design and computational evaluation of a multi-epitope vaccine for monkeypox virus: Insights into binding stability and immunological memory
- PMID: 39247273
- PMCID: PMC11380015
- DOI: 10.1016/j.heliyon.2024.e36154
Rational design and computational evaluation of a multi-epitope vaccine for monkeypox virus: Insights into binding stability and immunological memory
Abstract
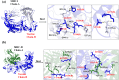
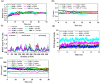
Multi-epitope vaccines strategically tackle rapidly mutating viruses by targeting diverse epitopes from different proteins, providing a comprehensive and adaptable immune protection approach for enhanced coverage against various viral variants. This research employs a comprehensive approach that includes the mapping of immune cells activating epitopes derived from the six structural glycoproteins (A29L, A30L, A35R, L1R, M1R, and E8L) of Monkeypox virus (Mpox). A total of 7 T-cells-specific epitopes, 13 B-cells-specific epitopes, and 5 IFN-γ activating epitopes were forecasted within these glycoproteins. The selection process focused on epitopes indicating high immunogenicity and favorable binding affinity with multiple MHC alleles. Following this, a vaccine has been formulated by incorporating the chosen epitopes, alongside adjuvants (PADRE peptide) and various linkers (EAAAK, GPGPG, and AAY). The physicochemical properties and 3D structure of the multi-epitope hybrid vaccine were analysed for characterization. MD simulations were employed to predict the binding stability between the vaccine and various pathogen recognition receptors such as TLRs (TLR1, TLR2, TLR4, and TLR6), as well as both class I and II MHC, achieved through hydrogen bonding and hydrophobic interactions. Through in silico cloning and immune simulation, it was observed that the multi-epitopes vaccine induced a robust memory immune response upon booster doses, forecasting protective immunity upon viral challenge. This protective immunity was characterized by the production of IgM + IgG antibodies, along with release of inflammatory cytokines like IFN-γ, and IL12, and the activation of various immune cells. This study offers valuable insights into the potential of a multi-epitope vaccine targeting the Mpox virus.
Keywords: Immune simulation; Immunoinformatics; MD simulation; Monkeypox virus; Multi-epitope hybrid vaccine.
© 2024 National Institute of Immunology.
Conflict of interest statement
Authors declare that they have no competing interests.
Figures
















References
-
- McCollum A.M., Damon I.K. Human monkeypox. Clin. Infect. Dis. 2014;58:260–267. https://academic.oup.com/cid/article/58/2/260/335791?login=true - PubMed
-
- Magnus P.V., Andersen E.K., Petersen K.B., Birch-Andersen A. A pox-like disease in Cynomolgus monkeys. Acta Pathol. Microbiol. Scand. 2009;46:156–176. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1699-0463.1959.tb00328.x - DOI
-
- Thornhill J.P., Barkati S., Walmsley S., Rockstroh J., Antinori A., Harrison L.B., Palich R., Nori A., Reeves I., Habibi M.S., Apea V., Boesecke C., Vandekerckhove L., Yakubovsky M., Sendagorta E., Blanco J.L., Florence E., Moschese D., Maltez F.M., Goorhuis A., Pourcher V., Migaud P., Noe S., Pintado C., Maggi F., Hansen A.B.E., Hoffmann C., Lezama J.I., Mussini C., Cattelan A.M., Makofane K., Tan D., Nozza S., Nemeth J., Klein M.B., Orkin C.M. Monkeypox virus infection in humans across 16 countries -April-June 2022. N. Engl. J. Med. 2022;387:679–691. https://www.nejm.org/doi/full/10.1056/NEJMoa2207323 - DOI - PubMed
-
- Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., Steffen R. The changing epidemiology of human monkeypox–a potential threat? A systematic review. PloS. Negl. Trop. Dis. 2022;16 https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0010141 - PMC - PubMed
-
- Nolasco S., Vitale F., Geremia A., Tramuto F., Maida C.M., Sciuto A., Coco C., Manuele R., Frasca E., Frasca M., Magliocco S., Gennaro A., Tumino E., Maresca M., Montineri A. First case of monkeypox virus, SARS-CoV-2 and HIV co-infection. J. Infect. 2023;1:e21–e23. https://www.sciencedirect.com/science/article/pii/S0163445322004790?via%... - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

