The clinical value of carbon nanoparticles in sentinel lymph node biopsy for early vulvar cancer
- PMID: 39247310
- PMCID: PMC11380014
- DOI: 10.1016/j.heliyon.2024.e36307
The clinical value of carbon nanoparticles in sentinel lymph node biopsy for early vulvar cancer
Abstract
Objective: Carbon nanoparticle (CNP)-guided sentinel lymph node biopsy (SLNB) has been extensively adopted as a cost-effective and highly efficient method for tracing malignant tumors except for those associated with vulvar cancer. The current study aimed to validate the feasibility and efficacy of CNPs in tracking sentinel lymph nodes (SLNs) in patients with early vulvar cancer.
Methods: We retrospectively reviewed patients with vulvar cancer at our institution from January 2016 to April 2022 who were pathologically diagnosed and underwent SLNB or inguinofemoral lymphadenectomy (IFLND). CNPs were the only lymphatic tracer used in SLNB. Patient demographics, perioperative outcomes and follow-up results, including overall survival (OS) and progression-free survival (PFS), were compared between the SLNB and IFLND groups.
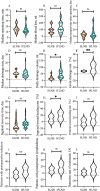
Results: Data from 52 patients were collected and investigated. Forty groins of 22 patients who underwent SLNB with CNP tracing were included. Black-stained SLNs were detected in 32 groins of 19 patients, and the rates of CNP detection by patient and by groin were 86.4 % and 80 %, respectively. Patients who underwent SLNB had better perioperative outcomes than those who underwent IFLND in certain aspects (groin drainage rate: 41.2 % and 80 %, respectively, p < 0.05; daily drainage volume (ml): 12.49 and 36.4, respectively, p < 0.05; and inguinal wound healing rate: 100 % and 80 %, respectively, p < 0.05). The results of survival analysis indicated similar prognoses for node-negative patients who underwent CNP-guided SLNB or IFLND.
Conclusions: Sentinel lymph node mapping with CNPs in vulvar cancer is feasible and demonstrates considerable biosecurity. With a satisfactory SLN detection rate achieved expediently, CNPs are a promising lymphatic tracer worthy of further utilization in vulvar cancer and could be an alternative option to canonical tracers.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
[Investigation of individualized treatment based on sentinel lymph node biopsy for early-stage vulvar cancer].Zhonghua Fu Chan Ke Za Zhi. 2015 Aug;50(8):596-602. Zhonghua Fu Chan Ke Za Zhi. 2015. PMID: 26675183 Chinese.
-
Sentinel lymph node biopsy procedure in squamous vulvar cancer. 10 years follow-up analysis.Rev Esp Med Nucl Imagen Mol (Engl Ed). 2020 Nov-Dec;39(6):360-366. doi: 10.1016/j.remn.2020.04.006. Epub 2020 Jun 17. Rev Esp Med Nucl Imagen Mol (Engl Ed). 2020. PMID: 32563714 English, Spanish.
-
[An initial experience of the use of sentinel lymph node biopsy in squamous cell cancer of the vulva].Zhonghua Fu Chan Ke Za Zhi. 2009 May;44(5):364-8. Zhonghua Fu Chan Ke Za Zhi. 2009. PMID: 19573313 Chinese.
-
Sentinel Lymph Node Evaluation in Early-Stage Vulvar Cancer.Curr Treat Options Oncol. 2024 Jan;25(1):20-26. doi: 10.1007/s11864-023-01165-1. Epub 2024 Jan 3. Curr Treat Options Oncol. 2024. PMID: 38170388 Review.
-
Sentinel node biopsy for diagnosis of lymph node involvement in endometrial cancer.Cochrane Database Syst Rev. 2021 Jun 9;6(6):CD013021. doi: 10.1002/14651858.CD013021.pub2. Cochrane Database Syst Rev. 2021. PMID: 34106467 Free PMC article.
References
-
- Woelber L., Mahner S., Voelker K., et al. Clinicopathological prognostic factors and patterns of recurrence in vulvar cancer. Anticancer Res. 2009;29(2):545–552. - PubMed
-
- Srinivas V., Morse M.J., Herr H.W., Sogani P.C., Whitmore W.F., Jr. Penile cancer: relation of extent of nodal metastasis to survival. J. Urol. 1987;137(5):880–882. - PubMed
-
- Sachdeva A., McGuinness L., Zapala L., et al. Management of lymph node-positive penile cancer: a systematic review. Eur. Urol. 2023;85(3):257–273. - PubMed
-
- National Comprehensive Cancer Network . NCCN; Fort Washington: 2022. NCCN Clinical Practice Guidelines in Oncology: Vulvar Cancer (Squamous Cell Carcinoma) (Version 1.2022)[EB/OL]http://www.nccn.org/ 2022-10-07.
-
- Burger M.P., Hollema H., Emanuels A.G., Krans M., Pras E., Bouma J. The importance of the groin node status for the survival of T1 and T2 vulval carcinoma patients. Gynecol. Oncol. 1995;57(3):327–334. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

