Mitral valve TEER in the UK: what you need to know as TEER becomes routinely available in the NHS
- PMID: 39247411
- PMCID: PMC11376252
- DOI: 10.5837/bjc.2023.034
Mitral valve TEER in the UK: what you need to know as TEER becomes routinely available in the NHS
Abstract
Transcatheter edge-to-edge repair (TEER) was first performed in 2003, and is now established across the developed world as an effective, minimally invasive treatment option for patients with mitral regurgitation (MR). Multiple large registries have established the efficacy of mitral TEER in patients with primary or degenerative MR in whom surgery is considered prohibitive or high risk, while ongoing randomised-controlled trials will determine its role in younger and lower- risk patients. In patients with secondary or functional MR, in whom mitral valve surgery is not routinely recommended, the pivotal COAPT trial showed a profound reduction in both mortality and heart failure hospitalisation in carefully selected patients. NHS England approved the routine commissioning of mitral TEER in 2019, and following a substantial delay, due in large part to the COVID pandemic, the procedure is now widely available across the UK. This review article describes the TEER procedure, currently available devices, the underlying evidence base, and the key facts needed for clinicians to understand who, how, and where to refer patients for consideration of mitral TEER. The emerging role of TEER in patients with severe symptomatic tricuspid regurgitation is also considered.
Keywords: mitral valve; percutaneous mitral valve leaflet repair; transcatheter edge-to-edge repair (TEER); tricuspid valve.
Copyright © 2023 Medinews (Cardiology) Limited.
Conflict of interest statement
Conflicts of interest DJB is a Consultant and Proctor for Abbott Vascular, Edwards Lifesciences, and Medtronic. SD is a Consultant and Proctor for Abbott Vascular and Edwards Lifesciences. RS is a Consultant and Proctor for Abbott Vascular. JB is a Consultant and Proctor for Abbott Vascular and Edwards Lifesciences. PAM is a Consultant and Proctor for Abbott Vascular and Edwards Lifesciences. DS none declared.
Figures

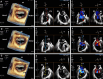
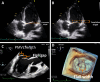
References
-
- NHS England. Clinical commissioning policy: percutaneous mitral valve leaflet repair for primary degenerative mitral regurgitation in adults. London: NHS England, July 2019. Available from: https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/201....
-
- National Institute for Health and Care Excellence. Percutaneous mitral valve leaflet repair for mitral regurgitation. IPG649. London: NICE, 2019. Available from: https://www.nice.org.uk/guidance/ipg649.
LinkOut - more resources
Full Text Sources
