STAT3 inhibitor Stattic Exhibits the Synergistic Effect with FGFRs Inhibitor Erdafitinib in FGFR1-positive Lung Squamous Cell Carcinoma
- PMID: 39247610
- PMCID: PMC11375536
- DOI: 10.7150/jca.97477
STAT3 inhibitor Stattic Exhibits the Synergistic Effect with FGFRs Inhibitor Erdafitinib in FGFR1-positive Lung Squamous Cell Carcinoma
Abstract
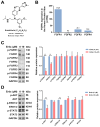
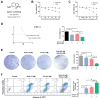
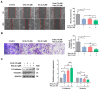
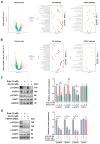
Lung squamous cell carcinoma (LUSC), a subset of non-small cell lung cancer (NSCLC), accounts for about 30% of all lung cancers (LC) and exhibits a dismal response to current therapeutic protocols. Existed studies have indicated that aberrations in fibroblast growth factor receptors (FGFRs) play a pivotal role in the progression of LUSC, rendering them as attractive targets for therapeutic intervention in this cancer type. This study found that Erdafitinib (Erda), a novel pan-FGF receptor tyrosine kinase inhibitor (TKI), exerted a cytotoxic effect on LUSC cells. However, STAT3, the downstream target of FGFRs, remained still activated despite Erdafitinib treatment. Then, a STAT3 inhibitor, Stattic (Sta), was concurrently used with Erdafitinib, and the combined treatment demonstrated a synergistic efficacy in both in vitro and in vivo models of LUSC when compared to that of the treatment of the Erdafitinib or Stattic alone. Further molecular studies showed that such an effect of Erdafitinib and Stattic was associated with their concurrently inhibitory effect on FGFR1 and STAT3 signaling in LUSC cells. Therefore, the findings of this study indicated that the concurrent use of Erdafitinib and Stattic is a promising therapeutic approach for the treatment of FGFR1-positive LUSC.
Keywords: Erdafitinib; FGFR1; Lung Squamous Cell Carcinoma; STAT3; Stattic.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Abu Rous F, Singhi EK, Sridhar A, Faisal MS, Desai A. Lung Cancer Treatment Advances in 2022. Cancer Invest. 2023;41(1):12–24. - PubMed
-
- Conti L, Gatt S. Squamous-Cell Carcinoma of the Lung. N Engl J Med. 2018;379(11):e17. - PubMed
-
- Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17(5):318–332. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

