Impact of SARS-CoV-2 Resistance to Antiviral Monoclonal Antibody Therapy on Neutralizing Antibody Response
- PMID: 39247686
- PMCID: PMC11378757
- DOI: 10.20411/pai.v9i2.718
Impact of SARS-CoV-2 Resistance to Antiviral Monoclonal Antibody Therapy on Neutralizing Antibody Response
Abstract
Background: Anti-SARS-CoV-2 monoclonal antibodies (mAbs) have played a key role as an anti-viral against SARS-CoV-2, but there is a potential for resistance to develop. The interplay between host antibody responses and the development of monoclonal antibody (mAb) resistance is a critical area of investigation. In this study, we assessed host neutralizing antibody (nAb) responses against both ancestral virus and those with treatment-emergent E484K bamlanivimab resistance mutations.
Methods: Study participants were enrolled in the ACTIV-2/Advancing Clinical Therapeutics Globally (ACTG) A5401 phase 2 randomized, placebo-controlled trial of bamlanivimab 700 mg mAb therapy (NCT04518410). Anterior nasal and nasopharyngeal swabs were collected for SARS-CoV-2 RNA testing and S gene next-generation sequencing to identify the E484K bamlanivimab resistance mutation. Serum nAb titers were assessed by pseudovirus neutralization assays.
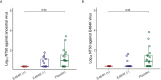
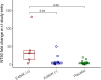
Results: Higher baseline (pre-treatment) nAb titers against either ancestral or E484K virus was associated with lower baseline viral load. Participants with emerging resistance had low levels of nAb titers against either ancestral or E484K nAb at the time of study entry. Participants with emergent E484K resistance developed significantly higher levels of E484K-specific nAb titers compared to mAb-treated individuals who did not develop resistance. All participants who developed the E484K mAb resistance mutation were eventually able to clear the virus.
Conclusion: Emerging drug resistance after SARS-CoV-2-specific mAb therapy led to a heightened host neutralizing antibody response to the mAb-resistant variant that was associated with eventual viral clearance. This demonstrates the interplay between the antiviral treatment-directed viral evolution and subsequent host immune response in viral clearance.
Keywords: COVID-19; SARS-CoV-2; bamlanivimab; monoclonal antibody; pseudovirus neutralization.
Copyright © 2024 Pathogens and Immunity.
Conflict of interest statement
UMP has consulted for Merck unrelated to the current work. JWM is a consultant for and receives grant support from Gilead Sciences, unrelated to the current work. JZL has consulted for Abbvie and received research support from Merck unrelated to the current work. KWC has consulted for Pardes Biosciences. DMS has consulted for Bayer Pharmaceuticals, Linear Therapies, Model Medicines, FluxErgy, and Vx Biosciences. ALL has consulted for Gilead and Abbott.
Figures

References
-
- Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Adams AC, Van Naarden J, Custer KL, Shen L, Durante M, Oakley G, Schade AE, Sabo J, Patel DR, Klekotka P, Skovronsky DM, Investigators B-. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N Engl J Med. 2021;384(3):229-37. doi: 10.1056/NEJMoa2029849. PubMed PMID: ; PMCID: . - DOI - PMC - PubMed
-
- Westendorf K, Zentelis S, Wang L, Foster D, Vaillancourt P, Wiggin M, Lovett E, van der Lee R, Hendle J, Pustilnik A, Sauder JM, Kraft L, Hwang Y, Siegel RW, Chen J, Heinz BA, Higgs RE, Kallewaard NL, Jepson K, Goya R, Smith MA, Collins DW, Pellacani D, Xiang P, de Puyraimond V, Ricicova M, Devorkin L, Pritchard C, O’Neill A, Dalal K, Panwar P, Dhupar H, Garces FA, Cohen CA, Dye JM, Huie KE, Badger CV, Kobasa D, Audet J, Freitas JJ, Hassanali S, Hughes I, Munoz L, Palma HC, Ramamurthy B, Cross RW, Geisbert TW, Menachery V, Lokugamage K, Borisevich V, Lanz I, Anderson L, Sipahimalani P, Corbett KS, Yang ES, Zhang Y, Shi W, Zhou T, Choe M, Misasi J, Kwong PD, Sullivan NJ, Graham BS, Fernandez TL, Hansen CL, Falconer E, Mascola JR, Jones BE, Barnhart BC.. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022;39(7):110812. doi: 10.1016/j.celrep.2022.110812. PubMed PMID: ; PMCID: . - DOI - PMC - PubMed
-
- Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, Sarkis E, Solis J, Zheng H, Scott N, Cathcart AL, Hebner CM, Sager J, Mogalian E, Tipple C, Peppercorn A, Alexander E, Pang PS, Free A, Brinson C, Aldinger M, Shapiro AE, Investigators C-I. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N Engl J Med. 2021;385(21):1941-50. doi: 10.1056/NEJMoa2107934. PubMed PMID: . - DOI - PubMed
-
- Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, Hebert C, Perry R, Boscia J, Heller B, Morris J, Crystal C, Igbinadolor A, Huhn G, Cardona J, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Dabora MC, Klekotka P, Shen L, Skovronsky DM, Investigators B-. Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19. N Engl J Med. 2021;385(15):1382-92. doi: 10.1056/NEJMoa2102685. PubMed PMID: ; PMCID: . - DOI - PMC - PubMed
-
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, Musser BJ, Soo Y, Rofail D, Im J, Perry C, Pan C, Hosain R, Mahmood A, Davis JD, Turner KC, Hooper AT, Hamilton JD, Baum A, Kyratsous CA, Kim Y, Cook A, Kampman W, Kohli A, Sachdeva Y, Graber X, Kowal B, DiCioccio T, Stahl N, Lipsich L, Braunstein N, Herman G, Yancopoulos GD, Trial I.. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med. 2021;384(3):238-51. doi: 10.1056/NEJMoa2035002. PubMed PMID: ; PMCID: . - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
