Reproductive tract complication risks following Chlamydia trachomatis infections: a long-term prospective cohort study from 2008 to 2022
- PMID: 39247903
- PMCID: PMC11378087
- DOI: 10.1016/j.lanepe.2024.101027
Reproductive tract complication risks following Chlamydia trachomatis infections: a long-term prospective cohort study from 2008 to 2022
Abstract
Background: The clinical and public health relevance of widespread testing for asymptomatic Chlamydia trachomatis (chlamydia) infections is under debate. To address uncertainties in screening programs, we estimate reproductive tract complication risks following asymptomatic and symptomatic chlamydia infections in a long-term prospective cohort.
Methods: A cohort of 5704 reproductive-age women recruited from a chlamydia screening study was followed for up to 14 years. Chlamydia positivity was determined using screening polymerase chain reaction test results, self-reported diagnoses (with/without symptoms), and chlamydia Immunoglobulin G antibodies. Outcome data (pregnancies, pelvic inflammatory disease (PID), ectopic pregnancy, and tubal factor infertility) were collected through self-completed questionnaires. Cox regression calculated adjusted hazard ratios (aHR) with confidence intervals (CI) to compare outcomes between time-updated chlamydia groups since sexual debut.
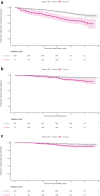
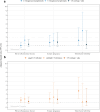
Findings: During 104,612 person-years, 2103 (36.9%) women were chlamydia-positive and 3692 women (64.7%) had been pregnant at least once. Risks for PID, ectopic pregnancy and tubal factor infertility were 1.62 (95% CI 1.20-2.17), 1.84 (95% CI 1.14-2.95) and 2.75 (95% CI 1.53-4.94), compared to chlamydia-negatives. aHRs for PID after symptomatic and asymptomatic infections were 2.29 (95% CI 1.62-3.25) and 1.06 (95% CI 0.66-1.69), respectively. Incidence of PID, ectopic pregnancy and tubal factor infertility after symptomatic chlamydia infection remained low with rates per 1000 person-years of 5.8, 1.9, and 1.8, respectively.
Interpretation: We found a significantly higher risk of PID, ectopic pregnancy and tubal factor infertility in chlamydia-positive women compared to chlamydia-negative women, although the overall incidence rates of complications remained low. Symptomatic, but not asymptomatic, chlamydia infections were associated with PID risk, suggesting the largest disease burden of complications is in this group.
Funding: The Netherlands Organisation for Health Research and Development (ZonMW Netherlands) and Research Funding from the Ministry of Health, Welfare and Sports.
Keywords: Asymptomatic chlamydia infections; Chlamydia control; Chlamydia trachomatis; Prospective cohort study; Reproductive tract complications.
© 2024 The Authors.
Conflict of interest statement
None declared.
Figures


References
-
- WHO . GenevaWorld Health Organization; Geneva: 2023. Fact sheet: sexually transmitted infections (STIs) world health organization.https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-in... Available from:
-
- Cantor A., Dana T., Griffin J.C., et al. Screening for chlamydial and gonococcal infections: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;326(10):957–966. - PubMed
-
- Hocking J.S., Temple-Smith M., Guy R., et al. Population effectiveness of opportunistic chlamydia testing in primary care in Australia: a cluster-randomised controlled trial. Lancet. 2018;392(10156):1413–1422. - PubMed
LinkOut - more resources
Full Text Sources

