Several factors that predict the outcome of large B-cell lymphoma patients who relapse/progress after chimeric antigen receptor (CAR) T-cell therapy can be identified before cell administration
- PMID: 39248284
- PMCID: PMC11382134
- DOI: 10.1002/cam4.70138
Several factors that predict the outcome of large B-cell lymphoma patients who relapse/progress after chimeric antigen receptor (CAR) T-cell therapy can be identified before cell administration
Abstract
Aim: The aim of this study was to analyse the outcomes of patients with large B-cell lymphoma (LBCL) treated with chimeric antigen receptor T-cell therapy (CAR-Tx), with a focus on outcomes after CAR T-cell failure, and to define the risk factors for rapid progression and further treatment.
Methods: We analysed 107 patients with LBCL from the Czech Republic and Slovakia who were treated in ≥3rd-line with tisagenlecleucel or axicabtagene ciloleucel between 2019 and 2022.
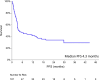
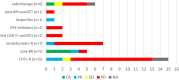
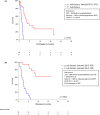
Results: The overall response rate (ORR) was 60%, with a 50% complete response (CR) rate. The median progression-free survival (PFS) and overall survival (OS) were 4.3 and 26.4 months, respectively. Sixty-three patients (59%) were refractory or relapsed after CAR-Tx. Of these patients, 39 received radiotherapy or systemic therapy, with an ORR of 22% (CR 8%). The median follow-up of surviving patients in whom treatment failed was 10.6 months. Several factors predicting further treatment administration and outcomes were present even before CAR-Tx. Risk factors for not receiving further therapy after CAR-Tx failure were high lactate dehydrogenase (LDH) levels before apheresis, extranodal involvement (EN), high ferritin levels before lymphodepletion (LD) and ECOG PS >1 at R/P. The median OS-2 (from R/P after CAR-Tx) was 6.7 months (6-month 57.9%) for treated patients and 0.4 months (6-month 4.2%) for untreated patients (p < 0.001). The median PFS-2 (from R/P after CAR-Tx) was 3.2 months (6-month 28.5%) for treated patients. The risk factors for a shorter PFS-2 (n = 39) included: CRP > limit of the normal range (LNR) before LD, albumin < LNR and ECOG PS > 1 at R/P. All these factors, together with LDH > LNR before LD and EN involvement at R/P, predicted OS-2 for treated patients.
Conclusion: Our findings allow better stratification of CAR-Tx candidates and stress the need for a proactive approach (earlier restaging, intervention after partial remission achievement).
Keywords: CAR T‐cell failure; outcomes of patients after CAR T‐cell therapy failure; relapsed/refractory large B‐cell lymphoma; risk factors for CAR T‐cell therapy failure.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Figures

References
-
- Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem‐cell transplantation for relapsed and refractory aggressive lymphomas: NCIC‐CTG LY.12. J Clin Oncol. 2014;32(31):3490‐3496. - PubMed
-
- Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B‐cell lymphoma who fail second‐line salvage regimens in the international CORAL study. Bone Marrow Transplant. 2016;51(1):51‐57. - PubMed
-
- Hwu P, Yang JC, Cowherd R, et al. In vivo antitumor activity of T cells redirected with chimeric antibody/T‐cell receptor genes. Cancer Res. 1995;55(15):3369‐3373. - PubMed
MeSH terms
Substances
Grants and funding
- NU21-03-00411/grant AZV NU21-03-00411 from the Ministry of Health of the Czech Republic
- This work was supported by the Ministry of Health, Czech Republic under grant MH CZ-DRO (UHHK, 00179906), under grant AZV NU21-03-00411 and by Charles University in Prague under the Cooperatio program, research area "Oncology and Haematology".
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

