Lebrikizumab Combined with Topical Corticosteroids Improves Patient-reported Outcomes in Japanese Patients with Moderate-to-severe Atopic Dermatitis
- PMID: 39248292
- PMCID: PMC11403364
- DOI: 10.2340/actadv.v104.34375
Lebrikizumab Combined with Topical Corticosteroids Improves Patient-reported Outcomes in Japanese Patients with Moderate-to-severe Atopic Dermatitis
Abstract
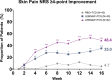
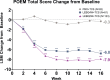
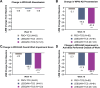
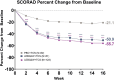
Lebrikizumab has previously demonstrated efficacy in Phase 3 trials: ADvocate1 and ADvocate2 (as monotherapy), ADhere, and ADhere-J (in combination with topical corticosteroids). Here, the impact of lebrikizumab combined with low- to mid-potency topical corticosteroids on patient-reported outcomes at 16 weeks in Japanese patients with moderate-to-severe atopic dermatitis is evaluated. Eligible patients (n = 286) were randomized 2:2:3 to receive placebo+ topical corticosteroids, 250 mg lebrikizumab every 4 weeks (LEBQ4W+topical corticosteroids, 500 mg loading dose at baseline), or 250 mg lebrikizumab every 2 weeks (LEBQ2W+ topical corticosteroids, 500 mg loading dose at baseline and Week 2) by subcutaneous injection. All PRO endpoints for the study were met; patients in the lebrikizumab in combination with topical corticosteroids groups demonstrated statistically significant and clinically meaningful improvements compared with placebo in combination with topical corticosteroids in Skin Pain NRS, DLQI, POEM, WPAI-AD, and SCORAD scales. Lebrikizumab combined with topical corticosteroids compared with placebo+topical corticosteroids improved patient-reported outcomes in Japanese patients with moderate-to-severe atopic dermatitis.
Conflict of interest statement
AK has received lecturer honoraria from AbbVie GK, Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc., Sanofi K.K., Taiho Pharmaceutical Co., Torii Pharmaceutical Co., Ltd., and Maruho Co., Ltd.; and has received research grants from Eli Lilly Japan K.K., Maruho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Taiho Pharmaceutical Co., Teijin Pharma Limited, and Torii Pharmaceutical Co., Ltd.. KI has received lecture fees from AbbVie GK, Eli Lilly Japan K.K., Maruho Co., Ltd., Pfizer Japan Inc., and Sanofi K.K. HT has no conflicts of interest to declare. RS has received lecturer honoraria from AbbVie GK, Eli Lilly Japan K.K., LEO Pharma, Maruho Co., Ltd., Pfizer Japan Inc., Sanofi K.K., and Torii Pharmaceutical Co., Ltd. YK has received lecturer honoraria from AbbVie GK, Pfizer Japan Inc., and Sanofi K.K., and research funding from AbbVie GK, Amgen, Eli Lilly Japan K.K., LEO Pharma, Maruho Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Sanofi K.K., and Taiho Pharmaceutical Co., Ltd. HT-I and YM are employees of Eli Lilly Japan K.K. SM is an employee of Eli Lilly and Company. NK Katoh has received honoraria as a speaker/consultant for AbbVie GK, Eli Lilly Japan K.K., LEO Pharma, Maruho Co., Ltd., Pfizer Japan Inc., Sanofi K.K., and Taiho Pharmaceutical Co., Ltd., and has received grants as an investigator from AbbVie GK, LEO Pharma, Maruho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and Torii Pharmaceutical Co., Ltd.
Figures

Similar articles
-
Efficacy and safety of lebrikizumab combined with topical corticosteroids in Japanese patients with moderate-to-severe atopic dermatitis: a phase 3, double-blind, placebo-controlled, randomized clinical trial (ADhere-J).Curr Med Res Opin. 2025 Jan;41(1):1-12. doi: 10.1080/03007995.2024.2436982. Epub 2024 Dec 13. Curr Med Res Opin. 2025. PMID: 39625230 Clinical Trial.
-
Efficacy of lebrikizumab in adolescent patients with moderate-to-severe atopic dermatitis: 16-week results from three randomized phase 3 clinical trials.J Dermatolog Treat. 2024 Dec;35(1):2324833. doi: 10.1080/09546634.2024.2324833. Epub 2024 May 12. J Dermatolog Treat. 2024. PMID: 38735650 Clinical Trial.
-
Improvement Across Dimensions of Disease with Lebrikizumab Use in Atopic Dermatitis: Two Phase 3, Randomized, Double-Blind, Placebo-Controlled Monotherapy Trials (ADvocate1 and ADvocate2).Adv Ther. 2025 Jan;42(1):132-143. doi: 10.1007/s12325-024-02974-y. Epub 2024 Sep 9. Adv Ther. 2025. PMID: 39249591 Free PMC article. Clinical Trial.
-
The role of lebrikizumab in the treatment of atopic dermatitis in the adult population.Immunotherapy. 2023 Sep;15(13):981-991. doi: 10.2217/imt-2023-0066. Epub 2023 Jul 4. Immunotherapy. 2023. PMID: 37401345 Review.
-
Tralokinumab in Atopic Dermatitis: A Profile of Its Use.Clin Drug Investig. 2022 Apr;42(4):365-374. doi: 10.1007/s40261-022-01135-9. Epub 2022 Mar 22. Clin Drug Investig. 2022. PMID: 35316850 Review.
References
-
- Katoh N, Saeki H, Kataoka Y, Etoh T, Teramukai S, Takagi H, et al. . Atopic dermatitis disease registry in Japanese adult patients with moderate to severe atopic dermatitis (ADDRESS-J): baseline characteristics, treatment history and disease burden. J Dermatol 2019; 46: 290–300. 10.1111/1346-8138.14787 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

