Phage-based biocontrol of Porphyromonas gingivalis through indirect targeting
- PMID: 39248462
- PMCID: PMC11497834
- DOI: 10.1128/aem.00951-24
Phage-based biocontrol of Porphyromonas gingivalis through indirect targeting
Abstract
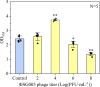
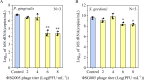
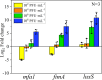
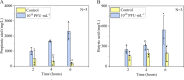
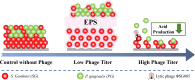
Bacteriophages offer an opportunity for chemical-free, precise control of problematic bacteria, but this approach can be limited when lytic phages are difficult to obtain for the target host. In such cases, phage-based targeting of cooperating or cross-feeding bacteria (e.g., Streptococcus gordonii) can be an effective approach to control the problematic bacteria (e.g., Porphyromonas gingivalis). Using a dual-species biofilm system, phage predation of S. gordonii (108 PFU·mL-1) decreased the abundance of pathogenic P. gingivalis by >99% compared with no-treatment controls, while also inhibiting the production of cytotoxic metabolic end products (butyric and propionic acids). Phage treatment upregulated genes associated with interspecies co-adhesion (5- to 8-fold) and quorum sensing (10-fold) in residual P. gingivalis, which is conducive to increased potential to bind to S. gordonii. Counterintuitively, lower-titer phage applications (104 PFU·mL-1) increased the production of extracellular polymeric substance (EPS) by 22% and biofilm biomass by 50%. This overproduction of EPS may contribute to the phenomenon where the biofilm separated into two distinct species layers, as observed by confocal laser scanning microscopy. Although more complex mixed-culture systems should be considered to delineate the merits and limitations of this novel biocontrol approach (which would likely require the use of phage cocktails), our results offer proof of concept that indirect phage-based targeting can expand the applicability of phage-based control of pathogenic bacteria for public health protection.
Importance: Lytic phages are valuable agents for targeted elimination of bacteria in diverse applications. Nevertheless, lytic phages are difficult to isolate for some target pathogens. We offer proof of concept that this limitation may be overcome via indirect phage targeting, which involves knocking out species that interact closely with and benefit the primary problematic target bacteria. Our target (P. gingivalis) only forms a periodontal pathogenic biofilm if the pioneer colonizer (S. gordonii) offers its surface for P. gingivalis to attach. Phage predation of the co-adhesive S. gordonii significantly reduced abundance of the target pathogen by >99%, decreased the total biofilm biomass by >44%, and suppressed its production of cytotoxic metabolic byproducts. Thus, this research extends the scope of phage-based biocontrol for public health protection.
Keywords: biofilm; indirect targeting; pathogen control; phage therapy; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Tobacco smoke augments Porphyromonas gingivalis-Streptococcus gordonii biofilm formation.PLoS One. 2011;6(11):e27386. doi: 10.1371/journal.pone.0027386. Epub 2011 Nov 14. PLoS One. 2011. PMID: 22110637 Free PMC article.
-
Natural antigenic differences in the functionally equivalent extracellular DNABII proteins of bacterial biofilms provide a means for targeted biofilm therapeutics.Mol Oral Microbiol. 2017 Apr;32(2):118-130. doi: 10.1111/omi.12157. Epub 2016 Apr 25. Mol Oral Microbiol. 2017. PMID: 26988714 Free PMC article.
-
[The impact of S.gordonii on P. gingivalis on the form of biofilm].Shanghai Kou Qiang Yi Xue. 2015 Dec;24(6):641-4. Shanghai Kou Qiang Yi Xue. 2015. PMID: 27063112 Chinese.
-
Biofilm control with natural and genetically-modified phages.World J Microbiol Biotechnol. 2016 Apr;32(4):67. doi: 10.1007/s11274-016-2009-4. Epub 2016 Mar 1. World J Microbiol Biotechnol. 2016. PMID: 26931607 Review.
-
Making waves: Intelligent phage cocktail design, a pathway to precise microbial control in water systems.Water Res. 2025 Jan 1;268(Pt A):122594. doi: 10.1016/j.watres.2024.122594. Epub 2024 Oct 9. Water Res. 2025. PMID: 39405620 Review.
References
-
- Hudson JA, McIntyre L, Billington C. 2010. Application of bacteriophages to control pathogenic and spoilage bacteria in food processing and distribution, p 119–135. In Bacteriophages in the control of food- and waterborne pathogens. doi:10.1128/9781555816629.ch7. - DOI
-
- Jia R, Unsal T, Xu D, Lekbach Y, Gu T. 2019. Microbiologically influenced corrosion and current mitigation strategies: a state of the art review. Int Biodeterior Biodegradation 137:42–58. doi:10.1016/j.ibiod.2018.11.007 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

