Flagellum-deficient Pseudomonas aeruginosa is more virulent than non-motile but flagellated mutants in a cystic fibrosis mouse model
- PMID: 39248473
- PMCID: PMC11448114
- DOI: 10.1128/spectrum.01325-24
Flagellum-deficient Pseudomonas aeruginosa is more virulent than non-motile but flagellated mutants in a cystic fibrosis mouse model
Abstract
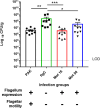
Loss of the flagellum marks the pathoadaptation of Pseudomonas aeruginosa to the cystic fibrosis (CF) airway environment during lung disease. Losing the flagellum is advantageous to the bacterium as the flagellum can be recognized by immune cells. The primary purpose of the flagellum is, however, to provide motility to the bacterium. Our goal was to determine whether the loss of flagellar motility or the loss of flagellum expression contributes to P. aeruginosa lung infection in CF. To address this, wild-type and gut-corrected FABP-human cystic fibrosis transmembrane conductance regulator (hCFTR) mice deficient in the murine Cftr gene were infected intratracheally with lethal doses of wild-type or flagellum-deficient P. aeruginosa. While there was no significant difference in the survival of wild-type mice after infection with either of the bacterial strains, a significantly higher mortality was observed in FABP-hCFTR mice infected with flagellum-deficient P. aeruginosa, compared to mice infected with their flagellated counterparts. When FABP-hCFTR mice were infected with isogenic, motility-deficient flagellated mutants, animal survival and lung bacterial titers were similar to those observed in mice infected with the wild-type bacterium. Airway levels of neutrophils and the amount neutrophil elastase were similar in mice infected with either the wild-type bacteria or the flagellum-deficient P. aeruginosa. Our results show that FABP-hCFTR mice have a different response to flagellum loss in P. aeruginosa compared to wild-type animals. The loss of flagellum expression, rather than the loss of motility, is the main driver behind the increased virulence of flagellum-deficient P. aeruginosa in CF. These observations provide new insight into P. aeruginosa virulence in CF.IMPORTANCEPseudomonas aeruginosa, a major respiratory pathogen in cystic fibrosis, is known to lose its flagellum during the course of infection in the airways. Here, we show that the loss of flagellum leads to a more enhanced virulence in Cftr-deficient cystic fibrosis mice than in control animals. Loss of flagellum expression, rather than the loss of flagellar swimming motility, represents the main driver behind this increased virulence suggesting that this appendage plays a specific role in P. aeruginosa virulence in cystic fibrosis airways.
Keywords: Pseudomonas aeruginosa; cystic fibrosis; flagellum; infection; inflammation; motility; neutrophil; neutrophil elastase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

