Tumor-Infiltrating Lymphocytes in Necrotic Tumors after Melanoma Neoadjuvant Anti-PD-1 Therapy Correlate with Pathologic Response and Recurrence-Free Survival
- PMID: 39248505
- PMCID: PMC11539852
- DOI: 10.1158/1078-0432.CCR-23-3775
Tumor-Infiltrating Lymphocytes in Necrotic Tumors after Melanoma Neoadjuvant Anti-PD-1 Therapy Correlate with Pathologic Response and Recurrence-Free Survival
Abstract
Purpose: Neoadjuvant anti-PD-1 therapy in melanoma may increase tumor-infiltrating lymphocytes (TIL), and more TIL are associated with better treatment response. A major pathologic response (MPR) in melanoma after neoadjuvant anti-PD-1 therapy usually comprises tumor necrosis and fibrosis. The role of TIL in necrotic tumor necrosis (nTIL) has not been explored.
Experimental design: We performed CD3 and CD8 IHC stains on 41 melanomas with geographic necrosis. Of the 41, 14 were immunotherapy-naïve, and 27 had been treated with one dose of neoadjuvant anti-PD-1 in two clinical trials. CD3+ and CD8+ nTIL were graded as absent/minimal or moderate/brisk. The percentage of necrotic areas in the tumor bed before and after treatment was quantified. The endpoints were MPR and 5-year recurrence-free survival (RFS).
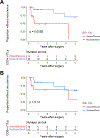
Results: In the immunotherapy-naïve cohort, 3/14 (21%) specimens had moderate/brisk CD3+, and 2/14 (14%) had moderate/brisk CD8+ nTIL. In the treated cohort, 16/27 (59%) specimens had moderate/brisk CD3+, and 15/27 (56%) had moderate/brisk CD8+ nTIL, higher than those of the naïve cohort (CD3, P = 0.046; CD8, P = 0.018). Tumor necrosis was significantly increased after anti-PD-1 therapy (P = 0.007). In the treated cohort, moderate/brisk CD3+ and CD8+ nTIL correlated with MPR (P = 0.042; P = 0.019, respectively). Treated patients with moderate/brisk CD3+ nTIL had higher 5-year RFS than those with absent/minimal nTIL (69% vs. 0%; P = 0.006). This persisted on multivariate analysis (HR, 0.16; 95% confidence interval, 0.03-0.84; P = 0.03), adjusted for pathologic response, which was borderline significant (HR, 0.26; 95% confidence interval, 0.07-1.01; P = 0.051).
Conclusions: CD3+ and CD8+ nTIL are associated with pathologic response and 5-year RFS in patients with melanoma after neoadjuvant anti-PD-1 therapy.
©2024 American Association for Cancer Research.
Conflict of interest statement
Figures

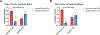
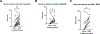
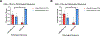
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

