Anti-Heat Shock Protein 70 Autoantibodies from Patients with Idiopathic Pulmonary Fibrosis Epigenetically Enhance Lung Fibroblast Apoptosis Resistance and Bcl-2 Expression
- PMID: 39248593
- PMCID: PMC11458357
- DOI: 10.4049/jimmunol.2400106
Anti-Heat Shock Protein 70 Autoantibodies from Patients with Idiopathic Pulmonary Fibrosis Epigenetically Enhance Lung Fibroblast Apoptosis Resistance and Bcl-2 Expression
Abstract
IgG autoantibodies to heat shock protein 70 (HSP70) are found in many immune-mediated clinical syndromes, and their presence among patients with idiopathic pulmonary fibrosis (IPF) portends especially poor outcomes. However, pathological effects of IPF anti-HSP70 have not been studied extensively. IPF lung fibroblasts are apoptosis resistant, and this dysregulation contributes to the accumulation of fibroblasts that characterizes the disease. During stress, HSP70 protein is exported extracellularly, where it binds to cognate cell surface receptors that mediate a variety of functional effects, including apoptosis inhibition. We hypothesized anti-HSP70 could engage HSP70-receptor complexes on fibroblasts that alter their apoptosis susceptibility. We found HSP70 is ubiquitously expressed on primary human lung fibroblasts. Treatment with anti-HSP70 isolated from patients with IPF with acute exacerbations increased Bcl-2 expression in human lung fibroblasts and reduced their susceptibility to staurosporine-induced apoptosis. Chromatin immunoprecipitation assays showed Bcl-2 gene promoter regions are enriched with the active histone mark H4 lysine 16 acetylation, and this was increased in the autoantibody-treated fibroblasts. When H4 lysine 16 acetylation was decreased by knocking down its acetyltransferase, MOF (males absent on the first), the anti-HSP70 treatments failed to upregulate Bcl-2. This study describes a heretofore unknown, to our knowledge, pathogenic consequence of autoimmunity in which autoantibodies affect the epigenetic regulation of fibroblast apoptosis. In addition to IPF, this autoimmune process could also have relevance in other immunological syndromes characterized by anti-HSP70 autoimmunity. These findings lend credence to the importance of autoimmunity in IPF and illustrate pathways that could be targeted in innovative therapies for this morbid, medically refractory lung disease.
Copyright © 2024 by The American Association of Immunologists, Inc.
Figures
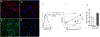
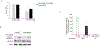


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

