A Framework for Quality Control in Quantitative Proteomics
- PMID: 39248652
- PMCID: PMC11973981
- DOI: 10.1021/acs.jproteome.4c00363
A Framework for Quality Control in Quantitative Proteomics
Abstract
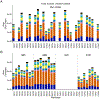
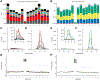
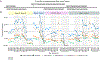
A thorough evaluation of the quality, reproducibility, and variability of bottom-up proteomics data is necessary at every stage of a workflow, from planning to analysis. We share vignettes applying adaptable quality control (QC) measures to assess sample preparation, system function, and quantitative analysis. System suitability samples are repeatedly measured longitudinally with targeted methods, and we share examples where they are used on three instrument platforms to identify severe system failures and track function over months to years. Internal QCs incorporated at the protein and peptide levels allow our team to assess sample preparation issues and to differentiate system failures from sample-specific issues. External QC samples prepared alongside our experimental samples are used to verify the consistency and quantitative potential of our results during batch correction and normalization before assessing biological phenotypes. We combine these controls with rapid analysis (Skyline), longitudinal QC metrics (AutoQC), and server-based data deposition (PanoramaWeb). We propose that this integrated approach to QC is a useful starting point for groups to facilitate rapid quality control assessment to ensure that valuable instrument time is used to collect the best quality data possible. Data are available on Panorama Public and ProteomeXchange under the identifier PXD051318.
Keywords: DDA; DIA; PRM; liquid chromatography; mass spectrometry; proteomics; quality control; quantitative results; sample preparation; system suitability.
Conflict of interest statement
Conflict of interest statement
The authors declare the following competing financial interest(s): The MacCoss Lab at the University of Washington has a sponsored research agreement with Thermo Fisher Scientific, the manufacturer of the instrumentation used in this research. M.J.M. is a paid consultant for Thermo Fisher Scientific. J.D.C. is an employee of Thermo Fisher Scientific
Figures




Update of
-
A framework for quality control in quantitative proteomics.bioRxiv [Preprint]. 2024 Aug 11:2024.04.12.589318. doi: 10.1101/2024.04.12.589318. bioRxiv. 2024. Update in: J Proteome Res. 2024 Oct 4;23(10):4392-4408. doi: 10.1021/acs.jproteome.4c00363. PMID: 38645098 Free PMC article. Updated. Preprint.
References
-
- Piehowski PD; Petyuk VA; Orton DJ; Xie F; Moore RJ; Ramirezrestrepo M; Engel A; Lieberman AP; Albin RL; Camp DG; Smith RD; Myers AJ Sources of Technical Variability in Quantitative LC - MS Proteomics: Human Brain Tissue Sample Analysis. J. Proteome Res 2013, 12, 2128–2137. 10.1021/pr301146m. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

