Plasma NfL, GFAP, amyloid, and p-tau species as Prognostic biomarkers in Parkinson's disease
- PMID: 39249107
- PMCID: PMC11588809
- DOI: 10.1007/s00415-024-12669-7
Plasma NfL, GFAP, amyloid, and p-tau species as Prognostic biomarkers in Parkinson's disease
Abstract
Introduction: The prognostic role of plasma neurofilament light chain (NfL), phospho-tau, beta-amyloid, and GFAP is still debated in Parkinson's disease (PD).
Methods: Plasma p-tau181, p-tau231, Aβ1-40, Aβ1-42, GFAP, and NfL were measured by SIMOA in 136 PD with 2.9 + 1.7 years of follow-up and 76 controls. Differences in plasma levels between controls and PD and their correlation with clinical severity and progression rates were evaluated using linear regression analyses.
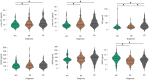
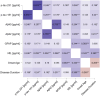
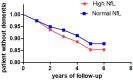
Results: Patients exhibited similar distribution of plasma biomarkers but higher P-tau181, P-tau231 and lower Aβ1-42 compared with controls. NfL and GFAP correlated with baseline motor and non-motor severity measures. At follow-up, NfL emerged as the best predictor of progression with marginal effect of GFAP and p-tau181 adjusting for age, sex, disease duration, and baseline motor severity.
Conclusion: The present findings confirmed plasma NfL as best predictor of progression in PD, with a marginal role of p-tau181 and GFAP.
Keywords: GFAP; Neurofilament light chain; Parkinson’s disease; Phosphorylated tau; Plasma biomarkers; Progression.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: All the authors report no disclosures related to this manuscript. Andrea Pilotto served in the advisory board of Z-cube (technology division of Zambon pharmaceuticals); he received honoraria from Z-cube s.r.l., Biomarin, Zambon, Nutricia and Chiesi Pharmaceuticals. He received research support from Vitaflo Germany and Zambon Italy. Nicholas Ashton has no financial conflicts to disclose. Alessandro Lupini has no financial conflicts to disclose. Beatrice Battaglio has no financial conflicts to disclose. Cinzia Zatti has no financial conflicts to disclose. Chiara Trasciatti has no financial conflicts to disclose. Stefano Gipponi has no financial conflicts to disclose. Elisabetta Cottini has no financial conflicts to disclose. Ilaria Grossi has no financial conflicts to disclose. Alessandro Salvi has no financial conflicts to disclose. Giuseppina De Petro has no financial conflicts to disclose. Marina Pizzi has no financial conflicts to disclose. Antonio Canale has no financial conflicts to disclose. Kaj Blennow has no financial conflicts to disclose. Henrik Zetterberg has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). Alessandro Padovani is consultant and served on the scientific advisory board of GE Healthcare, Eli-Lilly and Actelion Ltd Pharmaceuticals, received speaker honoraria from Nutricia, PIAM, Lansgstone Technology, GE Healthcare, Lilly, UCB Pharma, and Chiesi Pharmaceuticals. He is founded by Grant of M1.
Figures
References
-
- Fereshtehnejad S-M, Romenets SR, Anang JBM, Latreille V, Gagnon J-F, Postuma RB (2015) New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol 72:863–873 - PubMed
-
- Pilotto A, di Schiano Cola F, Premi E, Grasso R, Turrone R, Gipponi S et al (2019) Extrastriatal dopaminergic and serotonergic pathways in Parkinson’s disease and in dementia with Lewy bodies: a 123 I-FP-CIT SPECT study. Eur J Nucl Med Mol Imaging 46:1642–51 - PubMed
-
- Boylan LS, Chiò A (2019) Divining progression in Parkinson disease with a blood test: NfL. Neurology 93(11):471–472 - PubMed
MeSH terms
Substances
Grants and funding
- number NP 1471/Health and Wealth research grants 2016 of the University of Brescia
- ADSF-21-831381-C/AD Strategic Fund and the Alzheimer's Association
- PNRR-MAD-2022-12376110/PNRR-Health
- R01 AG068398/AG/NIA NIH HHS/United States
- JPND2019-466-236/European Union Joint Program for Neurodegenerative Disorders
- 2017-00915/Swedish Research Council
- 2017MYJ5TH/Ministero dell'Istruzione, dell'Università e della Ricerca
- JPND2021-00694/EU Joint Programme - Neurodegenerative Disease Research
- ADSF-21-831376-C/AD Strategic Fund and the Alzheimer's Association
- ADSF-21-831377-C/AD Strategic Fund and the Alzheimer's Association
- ALFGBG-715986/ALF-agreement
- ID853981/H2020 European Research Council
- UKDRI-1003/UCLH Biomedical Research Centre
- FO2017-0243/Hjärnfonden
- FO2022-0270/Stiftelsen för Gamla Tjänarinnor
- 2019-02397/Swedish Research Council
- DMA/Health and Wealth research grants 2016 of the University of Brescia
- AF-742881/Swedish Alzheimer Foundation
- AGYR2021/Airalzh Foundation
- ADDF/Alzheimer Drug Discovery Foundation
- P30 AG072980/AG/NIA NIH HHS/United States
- 101053962/HORIZON EUROPE Reforming and enhancing the European Research and Innovation system
- 860197 (MIRIADE)/H2020 Marie Skłodowska-Curie Actions
- RF-2018-12366209/Ministero della Salute
- 2022-01018/Swedish Research Council
- ALFGBG-71320/Swedish State Support for Clinical Research
- 1R01AG068398-01/Office of Dietary Supplements
- PRIN 2021/RePlast
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

