Safety of Rituximab biosimilar (Riximyo®) following a single switch from the reference product in patients with Non-Hodgkin's lymphoma: a retrospective study
- PMID: 39249493
- PMCID: PMC11534887
- DOI: 10.1007/s00277-024-05981-9
Safety of Rituximab biosimilar (Riximyo®) following a single switch from the reference product in patients with Non-Hodgkin's lymphoma: a retrospective study
Abstract
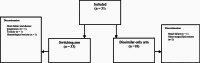
Unlike small molecule drugs and generic products, the active component of biologics and biosimilars are not identical chemical entities. Despite bioequivalence, there is limited evidence in clinical practice (i.e. Phase IV post-marketing surveillance) regarding the safety of biosimilar rituximab and even less so for "switching therapy" with respect to safety. Drug substitution by switching aims to realise cost savings by changing therapy involving a reference (biologic) product to a biosimilar. A retrospective analysis of safety outcomes including treatment-emergent adverse effects (TEAEs), rates of death and discontinuation of therapy, for all patients that received switching therapy (from reference to biosimilar rituximab, n = 33) was compared to patients who did not did not switch therapy (received biosimilar rituximab only, n = 18) at an Australian metropolitan cancer centre, over a six-month period. There was no statistical significant differences for any safety outcomes examined. Switching therapy for patients receiving rituximab does not lead to poorer safety outcomes.
Keywords: Biosimilars; GP2013; Rituximab; Safety; Switching.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Similar articles
-
Comparative Efficacy and Safety of Biosimilar Rituximab and Originator Rituximab in Rheumatoid Arthritis and Non-Hodgkin's Lymphoma: A Systematic Review and Meta-analysis.BioDrugs. 2019 Oct;33(5):469-483. doi: 10.1007/s40259-019-00376-z. BioDrugs. 2019. PMID: 31446557
-
Real-world use and acceptance of biosimilar monoclonal antibodies of rituximab in oncology practice in the USA.Future Oncol. 2021 Oct;17(30):3941-3950. doi: 10.2217/fon-2021-0618. Epub 2021 Jul 14. Future Oncol. 2021. PMID: 34259584
-
Safety of switching between rituximab biosimilars in onco-hematology.Sci Rep. 2021 Mar 16;11(1):5956. doi: 10.1038/s41598-021-85563-1. Sci Rep. 2021. PMID: 33727667 Free PMC article.
-
Real-world use and acceptance of rituximab biosimilars in non-Hodgkin lymphoma in an oncologist network in Germany.Future Oncol. 2020 May;16(15):1001-1012. doi: 10.2217/fon-2020-0180. Epub 2020 Apr 14. Future Oncol. 2020. PMID: 32286864
-
GP2013: A Rituximab Biosimilar.BioDrugs. 2017 Oct;31(5):465-468. doi: 10.1007/s40259-017-0245-2. BioDrugs. 2017. PMID: 28921160 Review.
References
-
- American Society of Clinical Oncology (ASCO) (2024) Lymphoma - Non-Hodgkin: Statistics. https://www.cancer.net/cancer-types/lymphoma-non-hodgkin/statistics (accessed 2nd
-
- Cai W, Zeng Q, Zhang X, Ruan W Trends Analysis of Non-Hodgkin Lymphoma at the National, Regional, and Global Level, 1990–2019: Results From the Global Burden of Disease Study 2019, (in English), Frontiers in Medicine, Original Research vol. 8, no. 1609, 2021-September-23 2021, 10.3389/fmed.2021.738693 - PMC - PubMed
-
- Dotan E, Aggarwal C, Smith MR (2010) Impact of Rituximab (Rituxan) on the Treatment of B-Cell Non-Hodgkin’s Lymphoma, (in eng), P & T: a peer-reviewed journal for formulary management, vol. 35, no. 3, pp. 148–157, [Online]. Available:https://pubmed.ncbi.nlm.nih.gov/20442809https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2844047/ - PMC - PubMed
-
- Pfreundschuh M et al (2006) CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group, (in eng), Lancet Oncol., vol. 7, no. 5, pp. 379 – 91, May 10.1016/s1470-2045(06)70664-7 - PubMed
-
- Tilly H et al (Sep 2015) Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up, (in eng). Ann Oncol 26(5):v116–v125. 10.1093/annonc/mdv304 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources