Elevated risk of adverse effects from foodborne contaminants and drugs in inflammatory bowel disease: a review
- PMID: 39249550
- PMCID: PMC11489187
- DOI: 10.1007/s00204-024-03844-w
Elevated risk of adverse effects from foodborne contaminants and drugs in inflammatory bowel disease: a review
Abstract
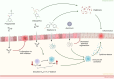
The global burden of Inflammatory bowel disease (IBD) has been rising over the last decades. IBD is an intestinal disorder with a complex and largely unknown etiology. The disease is characterized by a chronically inflamed gastrointestinal tract, with intermittent phases of exacerbation and remission. This compromised intestinal barrier can contribute to, enhance, or even enable the toxicity of drugs, food-borne chemicals and particulate matter. This review discusses whether the rising prevalence of IBD in our society warrants the consideration of IBD patients as a specific population group in toxicological safety assessment. Various in vivo, ex vivo and in vitro models are discussed that can simulate hallmarks of IBD and may be used to study the effects of prevalent intestinal inflammation on the hazards of these various toxicants. In conclusion, risk assessments based on healthy individuals may not sufficiently cover IBD patient safety and it is suggested to consider this susceptible subgroup of the population in future toxicological assessments.
Keywords: Chemically-induced disorders; Crohn’s disease; Drug-related side effects and adverse reactions; Foodborne illnesses; Inflammatory bowel disease; Ulcerative colitis.
© 2024. The Author(s).
Conflict of interest statement
TW, MB, JW, JD, EvdS, and NK declare no conflict of interests. HB is a board member of the Dutch Society of Toxicology, and a member of the Dutch Health Council. MD is the chair of the Dutch research organization Initiate on Crohn’s and Colitis (ICC).
Figures



References
-
- Alatab S, Sepanlou SG, Ikuta K, Vahedi H, Bisignano C, Safiri S, Sadeghi A, Nixon MR, Abdoli A, Abolhassani H et al (2020) The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 5(1):17–30. 10.1016/S2468-1253(19)30333-4 - DOI - PMC - PubMed
-
- Amirabadi HE, Donkers JM, Wierenga E, Ingenhut B, Pieters L, Stevens L, Donkers T, Westerhout J, Masereeuw R, Bobeldijk-Pastorova I et al (2022) Intestinal explant barrier chip: long-term intestinal absorption screening in a novel microphysiological system using tissue explants. Lab Chip 22(2):326–342. 10.1039/D1LC00669J - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

