Mycorrhizal symbiosis and the nitrogen nutrition of forest trees
- PMID: 39249589
- PMCID: PMC11384646
- DOI: 10.1007/s00253-024-13298-w
Mycorrhizal symbiosis and the nitrogen nutrition of forest trees
Abstract
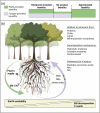
Terrestrial plants form primarily mutualistic symbiosis with mycorrhizal fungi based on a compatible exchange of solutes between plant and fungal partners. A key attribute of this symbiosis is the acquisition of soil nutrients by the fungus for the benefit of the plant in exchange for a carbon supply to the fungus. The interaction can range from mutualistic to parasitic depending on environmental and physiological contexts. This review considers current knowledge of the functionality of ectomycorrhizal (EM) symbiosis in the mobilisation and acquisition of soil nitrogen (N) in northern hemisphere forest ecosystems, highlighting the functional diversity of the fungi and the variation of symbiotic benefits, including the dynamics of N transfer to the plant. It provides an overview of recent advances in understanding 'mycorrhizal decomposition' for N release from organic or mineral-organic forms. Additionally, it emphasises the taxon-specific traits of EM fungi in soil N uptake. While the effects of EM communities on tree N are likely consistent across different communities regardless of species composition, the sink activities of various fungal taxa for tree carbon and N resources drive the dynamic continuum of mutualistic interactions. We posit that ectomycorrhizas contribute in a species-specific but complementary manner to benefit tree N nutrition. Therefore, alterations in diversity may impact fungal-plant resource exchange and, ultimately, the role of ectomycorrhizas in tree N nutrition. Understanding the dynamics of EM functions along the mutualism-parasitism continuum in forest ecosystems is essential for the effective management of ecosystem restoration and resilience amidst climate change. KEY POINTS: • Mycorrhizal symbiosis spans a continuum from invested to appropriated benefits. • Ectomycorrhizal fungal communities exhibit a high functional diversity. • Tree nitrogen nutrition benefits from the diversity of ectomycorrhizal fungi.
Keywords: Decomposition; Ectomycorrhizal functional traits; Mutualistic spectrum; Nitrogen cycle.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Arbuscular Mycorrhizal Tree Communities Have Greater Soil Fungal Diversity and Relative Abundances of Saprotrophs and Pathogens than Ectomycorrhizal Tree Communities.Appl Environ Microbiol. 2022 Jan 11;88(1):e0178221. doi: 10.1128/AEM.01782-21. Epub 2021 Oct 20. Appl Environ Microbiol. 2022. PMID: 34669435 Free PMC article.
-
Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees.Proc Natl Acad Sci U S A. 2016 Aug 2;113(31):8741-6. doi: 10.1073/pnas.1601006113. Epub 2016 Jul 18. Proc Natl Acad Sci U S A. 2016. PMID: 27432986 Free PMC article.
-
Greater carbon allocation to mycorrhizal fungi reduces tree nitrogen uptake in a boreal forest.Ecology. 2016 Apr;97(4):1012-22. doi: 10.1890/15-1222.1. Ecology. 2016. PMID: 27220217
-
Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: A review.Environ Pollut. 2019 Mar;246:148-162. doi: 10.1016/j.envpol.2018.11.074. Epub 2018 Nov 28. Environ Pollut. 2019. PMID: 30543941 Review.
-
Mycorrhizal types differ in ecophysiology and alter plant nutrition and soil processes.Biol Rev Camb Philos Soc. 2019 Oct;94(5):1857-1880. doi: 10.1111/brv.12538. Epub 2019 Jul 3. Biol Rev Camb Philos Soc. 2019. PMID: 31270944 Review.
Cited by
-
A Model of the Ectomycorrhizal Contribution to Forest Soil C and N Dynamics and Tree N Supply Within the EFIMOD3 Model System.Plants (Basel). 2025 Jan 31;14(3):417. doi: 10.3390/plants14030417. Plants (Basel). 2025. PMID: 39942979 Free PMC article.
-
Mycorrhizal Fungi Influence on Mature Tree Growth: Stronger in High-Nitrogen Soils for an EMF-Associated Tree and in Low-Nitrogen Soils for Two AMF-Associated Trees.Plant Environ Interact. 2025 May 8;6(3):e70055. doi: 10.1002/pei3.70055. eCollection 2025 Jun. Plant Environ Interact. 2025. PMID: 40342515 Free PMC article.
References
-
- Agerer R (2001) Exploration types of ectomycorrhizae. Mycorrhiza 11:107–114. 10.1007/s005720100108
-
- Ågren GI, Hyvönen R, Baskaran P (2019) Ectomycorrhiza, friend or foe? Ecosystems 22:1561–1572. 10.1007/s10021-019-00356-y
-
- Almario J, Fabiańska I, Saridis G, Bucher M (2022) Unearthing the plant–microbe quid pro quo in root associations with beneficial fungi. New Phytol 234:1967–1976. 10.1111/nph.18061 - PubMed
-
- Anthony MA, Crowther TW, van der Linde S, Suz LM, Bidartondo MI, Cox F, Schaub M, Rautio P, Ferretti M, Vesterdal L, De Vos B, Dettwiler M, Eickenscheidt N, Schmitz A, Meesenburg H, Andreae H, Jacob F, Dietrich H-P, Waldner P, Gessler A, Frey B, Schramm O, van den Bulk P, Hensen A, Averill C (2022) Forest tree growth is linked to mycorrhizal fungal composition and function across Europe. ISME J 16:1327–1336. 10.1038/s41396-021-01159-7 - PMC - PubMed
-
- Argiroff WA, Zak DR, Pellitier PT, Upchurch RA, Belke JP (2022) Decay by ectomycorrhizal fungi couples soil organic matter to nitrogen availability. Ecol Lett 25:391–404. 10.1111/ele.13923 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

