NLRP1-dependent activation of Gasdermin D in neutrophils controls cutaneous leishmaniasis
- PMID: 39250503
- PMCID: PMC11412672
- DOI: 10.1371/journal.ppat.1012527
NLRP1-dependent activation of Gasdermin D in neutrophils controls cutaneous leishmaniasis
Abstract
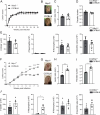
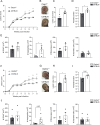
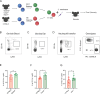
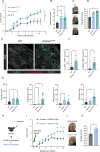
Intracellular pathogens that replicate in host myeloid cells have devised ways to inhibit the cell's killing machinery. Pyroptosis is one of the host strategies used to reduce the pathogen replicating niche and thereby control its expansion. The intracellular Leishmania parasites can survive and use neutrophils as a silent entry niche, favoring subsequent parasite dissemination into the host. Here, we show that Leishmania mexicana induces NLRP1- and caspase-1-dependent Gasdermin D (GSDMD)-mediated pyroptosis in neutrophils, a process critical to control the parasite-induced pathology. In the absence of GSDMD, we observe an increased number of infected dermal neutrophils two days post-infection. Using adoptive neutrophil transfer in neutropenic mice, we show that pyroptosis contributes to the regulation of the neutrophil niche early after infection. The critical role of neutrophil pyroptosis and its positive influence on the regulation of the disease outcome was further demonstrated following infection of mice with neutrophil-specific deletion of GSDMD. Thus, our study establishes neutrophil pyroptosis as a critical regulator of leishmaniasis pathology.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

