Serological Markers of Clinical Improvement in MuSK Myasthenia Gravis
- PMID: 39250722
- PMCID: PMC11385952
- DOI: 10.1212/NXI.0000000000200313
Serological Markers of Clinical Improvement in MuSK Myasthenia Gravis
Abstract
Background and objectives: In this retrospective longitudinal study, we aimed at exploring the role of (a) MuSK-immunoglobulin G (IgG) levels, (b) predominant MuSK-IgG subclasses, and (c) antibody affinity as candidate biomarkers of severity and outcomes in MuSK-MG, using and comparing different antibody testing techniques.
Methods: Total MuSK-IgGs were quantified with radioimmunoassay (RIA), ELISA, flow cytometry, and cell-based assay (CBA) serial dilutions using HEK293 cells transfected with MuSK-eGFP. MuSK-IgG subclasses were measured by flow cytometry. SAffCon assay was used for determining MuSK-IgG affinity.
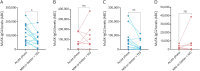
Results: Forty-three serum samples were obtained at different time points from 20 patients with MuSK-MG (median age at onset: 48 years, interquartile range = 27.5-72.5; women, 16/20), with 9 of 20 (45%) treated with rituximab. A strong correlation between MuSK-IgG levels measured by flow cytometry and RIA titers was found (rs = 0.74, 95% CI 0.41-0.89, p = 0.0003), as well as a moderate correlation between CBA end-point titers and RIA titers (rs = 0.47, 95% CI 0.01-0.77, p = 0.0414). A significant correlation was found between MuSK-IgG flow cytometry levels and disease severity (rs = 0.39, 95% CI 0.06-0.64, p = 0.0175; mixed-effects model estimate: 2.296e-06, std. error: 1.024e-06, t = 2.243, p = 0.032). In individual patients, clinical improvement was associated with decrease in MuSK-IgG levels, as measured by either flow cytometry or CBA end-point titration. In all samples, MuSK-IgG4 was the most frequent isotype (mean ± SD: 90.95% ± 13.89). A significant reduction of MuSK-IgG4 and, to a lesser extent, of MuSK-IgG2, was seen in patients with favorable clinical outcomes. A similar trend was confirmed in the subgroup of rituximab-treated patients. In a single patient, MuSK-IgG affinity increased during symptom exacerbation (KD values: 62 nM vs 0.6 nM) while total MuSK-IgG and IgG4 levels remained stable, suggesting that affinity maturation may be a driver of clinical worsening.
Discussion: Our data support the quantification of MuSK antibodies by flow cytometry. Through a multimodal investigational approach, we showed that total MuSK-IgG levels, MuSK-IgG4 and MuSK-IgG2 levels, and MuSK-IgG affinity may represent promising biomarkers of disease outcomes in MuSK-MG.
Conflict of interest statement
G. Spagni is supported by the European Academy of Neurology Research Training Fellowship. Bo Sun is supported by an NIHR Academic Clinical Lectureship (CL-2021-13-002), The Academy of Medical Sciences and the British Medical Association Foundation. Valentina Damato is supported by the Myasthenia Gravis Rare Disease Network-MGNet, a member of the Rare Disease Clinical Research Network Consortium (RDCRN) NIH U54 NS115054. All RDCRN consortia are supported by the network's Data Management and Coordinating Center (DMCC) (U2CTR002818), by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006) - A Multiscale integrated approach to the study of the nervous system in health and disease (DR. 1553 11.10.2022), by a grant “Giovani Ricercatori - Ricerca Finalizzata 2021” code GR-2021-12375527 (“NEURO-CHECKMATE”) from the Italian Ministry of Health. Go to
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
