Estimating the burden of vaccine-preventable lower respiratory tract disease in UK primary care: protocol for a prospective surveillance study (AvonCAP GP2)
- PMID: 39251234
- PMCID: PMC11687260
- DOI: 10.3399/BJGPO.2024.0129
Estimating the burden of vaccine-preventable lower respiratory tract disease in UK primary care: protocol for a prospective surveillance study (AvonCAP GP2)
Abstract
Background: The true burden of acute lower respiratory tract disease (aLRTD; includes acute lower respiratory tract infection [aLRTI] and presumed non-infective exacerbations of chronic lung disease and heart failure) among adults presenting to primary care, and the proportion that are potentially vaccine preventable is unknown.
Aim: To describe aLRTD incidence in adults presenting to primary care; estimate proportions caused by respiratory syncytial virus (RSV), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and Streptococcus pneumoniae (SP); and investigate disease burden from patient and NHS perspectives.
Design & setting: Primary care prospective cohort study conducted in six representative general practices (total ∼86 000 registered adults) in Bristol, UK.
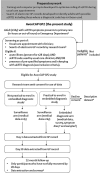
Method: Adults (aged ≥18 years) registered at participating general practices and presenting to primary care (in-hours or out-of-hours) or emergency department (if not admitted) with aLRTD will be eligible. They will be identified by real-time primary care record searches. Researchers will screen electronic GP records, including free text, contact patients to assess eligibility, and offer enrolment in a surveillance study and an enhanced diagnostic study (urine, saliva, and respiratory samples; physical examination; and symptom diaries). Data will be collected for all aLRTD episodes, with patients assigned to one of three arms: surveillance; embedded diagnostic; and descriptive dataset. Outcome measures will include clinical and pathogen-defined aLRTD incidence rates, symptom severity and duration, NHS contacts and costs, health-related quality-of-life changes, and mortality (≤30 days post-identification).
Conclusion: This comprehensive surveillance study of adults presenting to primary care with aLRTD, with embedded detailed data and sample collection, will provide an accurate assessment of aLRTD burden due to vaccine-preventable infections.
Keywords: lower respiratory tract infection; primary health care; respiratory syncytial viruses; respiratory tract diseases.
Copyright © 2024, The Authors.
Conflict of interest statement
This study will be conducted as a university-guided collaboration between the University of Bristol (sponsor) and Pfizer (funder). AF is a member of the Joint Committee on Vaccination and Immunisation (JCVI) subcommittees on pneumococcal and respiratory syncytial virus vaccines. In addition to receiving funding from Pfizer as Chief Investigator of this study, AF leads another project investigating transmission of respiratory bacteria in families jointly funded by Pfizer and the Gates Foundation. Authors with Pfizer affiliations are employed by Pfizer and may own Pfizer stock. All other authors have declared no competing interests.
Figures
References
-
- Hyams C, Begier E, Garcia Gonzalez M, et al. Incidence of acute lower respiratory tract disease hospitalisations, including pneumonia, among adults in Bristol, UK, 2019, estimated using both a prospective and retrospective methodology. BMJ Open. 2022; 12 (6):e057464. doi: 10.1136/bmjopen-2021-057464. - DOI - PMC - PubMed
-
- European Respiratory Society The European Lung White Book. 2013. [3 Dec 2024]. The economic burden of lung disease. accessed.
LinkOut - more resources
Full Text Sources
Miscellaneous
