Niraparib in Japanese patients with heavily pretreated, homologous recombination-deficient ovarian cancer: final results of a multicenter phase 2 study
- PMID: 39251349
- PMCID: PMC11390254
- DOI: 10.3802/jgo.2024.35.e114
Niraparib in Japanese patients with heavily pretreated, homologous recombination-deficient ovarian cancer: final results of a multicenter phase 2 study
Abstract
Objective: To evaluate the long-term efficacy and safety of niraparib in Japanese women with heavily pretreated ovarian cancer.
Methods: This was the follow-up analysis of a phase 2, multicenter, open-label, single-arm study in Japanese women with homologous recombination-deficient, platinum-sensitive, relapsed, high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer who had completed 3-4 lines of chemotherapy and were poly(ADP-ribose) polymerase inhibitor naïve. Participants received niraparib (starting dose, 300 mg) once daily in continuous 28-day cycles until objective disease progression, unacceptable toxicity, or consent withdrawal. The primary endpoint was confirmed objective response rate (ORR), as assessed using Response Evaluation Criteria in Solid Tumors version 1.1. Safety evaluations included treatment-emergent adverse events (TEAEs).
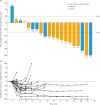
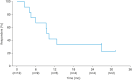
Results: 20 patients were enrolled in the study and included in both efficacy and safety analyses. Median total study duration was 759.5 days. Median dose intensity was 201.3 mg/day. Confirmed ORR was 60.0% (90% confidence interval [CI]=39.4-78.3); 2 patients had complete response and 10 patients had partial response. Median duration of response was 9.9 months (95% CI=3.9-26.9) and the disease control rate was 90.0% (95% CI=68.3-98.8). The most common TEAEs were anemia (n=15), nausea (n=12), and decreased platelet count (n=11). TEAEs leading to study drug dose reduction, interruption, or discontinuation were reported in 16 (80.0%), 15 (75.0%), and 2 patients (10.0%), respectively.
Conclusion: The long-term efficacy and safety profile of niraparib was consistent with previous findings in the equivalent population in non-Japanese patients. No new safety signals were identified.
Trial registration: ClinicalTrials.gov Identifier: NCT03759600.
Keywords: Clinical Trial, Phase II; Ovarian Cancer; Poly(ADP-ribose) Polymerase Inhibitors.
© 2024. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology, and Japan Society of Gynecologic Oncology.
Conflict of interest statement
Daisuke Aoki declares the receipt of consulting fees from AstraZeneca, Chugai, MSD, and Takeda Pharmaceutical Company Ltd, and honoraria from AstraZeneca, Chugai, MSD, Myriad Genetics, and Takeda Pharmaceutical Company Ltd. Tsutomu Tabata declares the receipt of lecture fees from AstraZeneca, Chugai, and Takeda Pharmaceutical Company Ltd. Satoshi Yanagida, Toshiaki Nakamura and Hidekatsu Nakai declare the receipt of lecture fees from Takeda Pharmaceutical Company Ltd. Kenichi Harano declares the receipt of grants from AstraZeneca, Chugai, Daiichi Sankyo, MSD, and Takeda Pharmaceutical Company Ltd, and speaker fees from Astra Zeneca, Chugai, Eisai, MSD, Taiho, and Takeda Pharmaceutical Company Ltd. Kenichi Harano also declares an advisory role for AstraZeneca, Chugai, Taiho and Takeda Pharmaceutical Company Ltd, and receipt of institutional research funding from Takeda Pharmaceutical Company Ltd. Kosei Hasegawa declares the receipt of research grants from Daiichi Sankyo, Eisai, MSD, and Takeda Pharmaceutical Company Ltd, honoraria from AstraZeneca, Chugai, Daiichi Sankyo, Eisai, Genmab, Kaken, Kyowa Kirin, MSD, Sanofi, and Takeda Pharmaceutical Company Ltd, and travel expenses from Regeneron. Kosei Hasegawa is also on the advisory board of Chugai, Eisai, Genmab, MSD, Roche, Sanofi, and Takeda Pharmaceutical Company Ltd. Shinichi Komiyama declares the receipt of consulting fees from AstraZeneca, Chugai, Daiichi Sankyo, Eisai, and MSD, and honoraria from AstraZeneca, Chugai, Eisai, and Takeda Pharmaceutical Company Ltd. Kazuhiro Takehara declares the receipt of speaker bureaus fees from AstraZeneca, MSD, and Takeda Pharmaceutical Company Ltd, and manuscript writing fees from MSD. Yoichi Kase and Ai Kato are employees of, and hold stocks in Takeda Pharmaceutical Company Ltd. Shuuji Sumino and Junpei Soeda are employees of Takeda Pharmaceutical Company Ltd. Ajit Suri is an employee of Millennium Pharmaceuticals, part of Takeda, and holds stocks in Takeda Pharmaceutical Company Ltd. Aikou Okamoto declares the receipt of honoraria from ASKA Pharmaceutical Co., Ltd, AstraZeneca, Bayer Holding Ltd, Chugai, Covidien Japan Inc., Eisai, Fuji Pharma Co., Ltd, Johnson & Johnson K.K., Kaken Pharmaceutical Co., Ltd, Kissei Pharmaceutical Co., Ltd, MSD, Mochida Pharmaceutical Co., Ltd, Myriad Genetics G.K., Otsuka Pharmaceutical Co., Ltd, Sanofi, Takeda Pharmaceutical Company Ltd, and Zeria Pharmaceutical Co., Ltd. Aikou Okamoto also declares the receipt of institutional grants from ASKA Pharmaceutical Co., Ltd, AstraZeneca, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Fuji Pharma Co., Ltd, Gyne Mom Co., Ltd, Kaken Pharmaceutical Co., Ltd, Linical Co., Ltd, Meiji Holdings Co., Ltd, Merck BioPharma Japan, Mochida Pharmaceutical Co., Ltd, MSD, Nippon Shinyaku Co., Ltd, Taiho Pharmaceutical Co., Ltd, and Tsumura & Co. Other authors have no conflict of interest to disclose.
Figures

References
-
- Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:636–648. - PubMed
-
- Sachdev E, Tabatabai R, Roy V, Rimel BJ, Mita MM. PARP inhibition in cancer: an update on clinical development. Target Oncol. 2019;14:657–679. - PubMed
-
- Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. - PubMed

