Risk of neuropsychiatric adverse events associated with montelukast use in children and adolescents: a population-based case-crossover study
- PMID: 39251365
- PMCID: PMC11733910
- DOI: 10.1136/bmjpo-2023-002483
Risk of neuropsychiatric adverse events associated with montelukast use in children and adolescents: a population-based case-crossover study
Abstract
Purpose: Montelukast is used extensively in children and adolescents for allergic rhinitis and asthma. However, concerns have been raised regarding the increased risk of neuropsychiatric adverse events (NPAEs) associated with montelukast use. Therefore, our case-crossover study was conducted to observe whether there is an increased risk of NPAEs associated with montelukast use in children and adolescents.
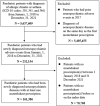
Materials and methods: A population-based case-crossover study using the customised Health Insurance Review and Assessment (HIRA) dataset was conducted. Paediatric patients aged between 0 and 19 years diagnosed with allergic rhinitis and/or asthma with a history of at least one montelukast prescription between 1 January 2018 and 31 December 2021 were included. Exposure to montelukast was assessed during 3-, 7-, 14-, 28- and 56-day hazard periods prior to each patient's NPAE. Stratified analyses according to age group, gender and season for the risk of NPAEs associated with montelukast use in the previous 7 days and 14 days were performed, respectively. Conditional logistic regression analysis was used to calculate adjusted ORs (aORs) with their corresponding 95% CIs, adjusting for concomitant medications.
Results: A total of 161 386 paediatric patients was identified. An increased risk of NPAEs associated with montelukast was found in all time window periods, including 3-day (aOR 1.28, 95% CI 1.24 to 1.32), 7-day (aOR 1.29, 95% CI 1.26 to 1.33), 14-day (aOR 1.34, 95% CI 1.31 to 1.37), 28-day (aOR 1.38, 95% CI 1.36 to 1.41) and 56-day (aOR 1.21, 95% CI 1.19 to 1.22) preceding hazard periods compared with use in the four control periods.
Conclusion: Children and adolescents with allergic rhinitis and/or asthma should be prescribed montelukast with caution considering clinical benefits.
Keywords: adolescent health; child psychiatry; infant; pharmacology.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None.
Figures

References
-
- Drug Safety Communications FDA requires Boxed Warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair) FDA (US) 2020
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
