Gut microbiome influences efficacy of Endostatin combined with PD-1 blockade against colorectal cancer
- PMID: 39251538
- PMCID: PMC11383918
- DOI: 10.1186/s43556-024-00200-3
Gut microbiome influences efficacy of Endostatin combined with PD-1 blockade against colorectal cancer
Abstract
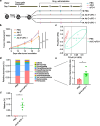
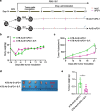
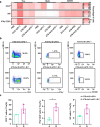
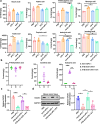
The combination of anti-angiogenic drugs and immune checkpoint inhibitors (ICIs) in the treatment of tumors is emerging as a way to improve ICIs-resistant tumor therapy. In addition, gut microbes (GMs) are involved in angiogenesis in the tumor microenvironment and are also associated with the antitumor function of immune checkpoint inhibitors. However, it is unclear whether gut microbes have a role in anti-tumor function in the combination of anti-angiogenic drugs and immune checkpoint inhibitors for cancer treatment. Endostatin, an angiogenesis inhibitor, has been widely used as an antiangiogenic therapy for cancer. We showed that combined therapy with an adenovirus encoding human endostatin, named Ad-E, and PD-1 blockade dramatically abrogated MC38 tumor growth. The structure of intestinal microbes in mice was changed after combination treatment. We found that the antitumor function of combination therapy was inhibited after the elimination of intestinal microbes. In mice with depleted microbiota, oral gavage of Bacteroides fragilis salvaged the antitumor effects of combination Ad-E and αPD-1 monoclonal antibody (mAb) to a certain extent. Further, Bacteroides fragilis could improve CD3+T cells, NK cells, and IFNγ+CD8+ T cells in the tumor microenvironment to inhibit tumor growth. Besides, Bacteroides fragilis might restore antitumor function by down-regulating isobutyric acid (IBA). Our results suggested that GMs may be involved in the combination of Ad-E and αPD-1 mAb for cancer treatment, which has oncological implications for tumor growth dynamics and cancer immune surveillance.
Keywords: Adenovirus encoding human endostatin; Anti-angiogenic therapy; Cancer therapy; Gut microbes; PD-1 blockade.
© 2024. The Author(s).
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures


References
-
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol. 2019;5:1411–20. 10.1001/jamaoncol.2019.2187. 10.1001/jamaoncol.2019.2187 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
