Gating elements for carvacrol activation of the OTOP1 proton channel
- PMID: 39251752
- PMCID: PMC11384762
- DOI: 10.1038/s42003-024-06818-x
Gating elements for carvacrol activation of the OTOP1 proton channel
Abstract
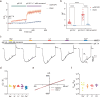
Otopetrin 1 (OTOP1) is a proton-activated channel crucial for animals' perception of sour taste. Despite its significance, the gating mechanism of OTOP1 remains poorly understood. Here, we demonstrate that carvacrol activates the mouse OTOP1 (mOTOP1) channel under neutral and acidic conditions. Functional analysis showed that carvacrol enhances pH fluorescence signals in OTOP1-expressing cells, with reduced efficacy at lower pH levels. Carvacrol selectively activates mOTOP1, while mOTOP2, mOTOP3, and Chelonia mydas OTOP1 (CmOTOP1) are insensitive to carvacrol activation under neutral pH. Through chimera and point mutation experiments, swapping S134 in transmembrane segment 3 (TM3) and T247 in the TM5-6 linker abolished carvacrol activation of mOTOP1 and conferred activation on CmOTOP1, suggesting these two residues are critical for carvacrol sensitivity. These findings highlight TM3 and TM5-6 linker as pivotal gating apparatus of OTOP1 channels and potential docking sites for drug design.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

